An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter11: The Chemical Elements
Section11.4: The Periodic Table
Problem 11.1CE
Related questions
Question
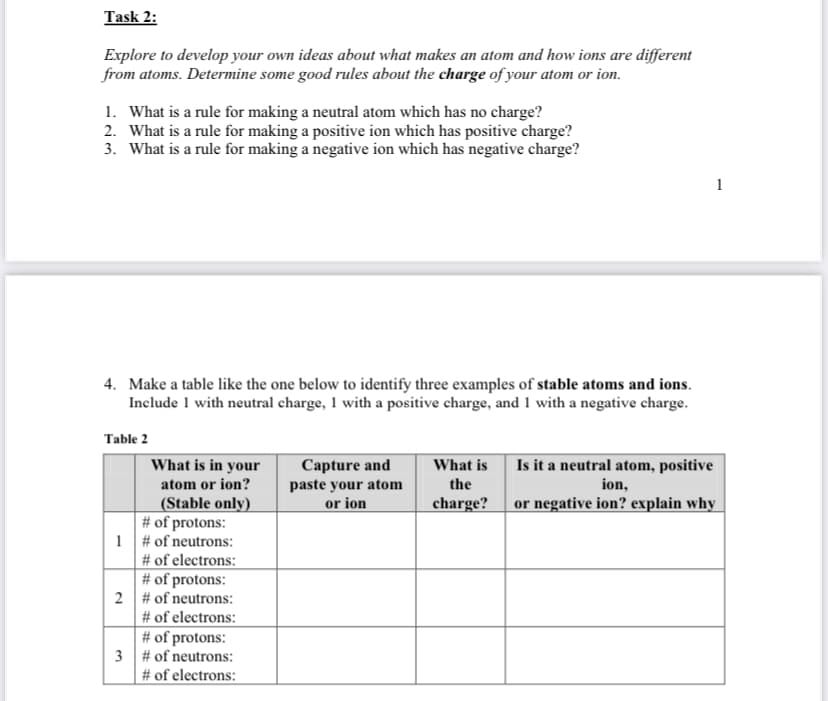
Transcribed Image Text:Task 2:
Explore to develop your own ideas about what makes an atom and how ions are different
from atoms. Determine some good rules about the charge of your atom or ion.
1. What is a rule for making a neutral atom which has no charge?
2. What is a rule for making a positive ion which has positive charge?
3. What is a rule for making a negative ion which has negative charge?
1
4. Make a table like the one below to identify three examples of stable atoms and ions.
Include 1 with neutral charge, 1 with a positive charge, and 1 with a negative charge.
Table 2
Is it a neutral atom, positive
Capture and
paste your atom
or ion
What is in your
What is
atom or ion?
the
ion,
or negative ion? explain why
(Stable only)
# of protons:
1 # of neutrons:
# of electrons:
# of protons:
2 # of neutrons:
charge?
# of electrons:
# of protons:
3 # of neutrons:
# of electrons:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

