Temperature (°C) Vapor pressure (Pa) Saturation vapor density (g/m³) 60 1.99 x 10' 130 70 3.12 x 104 197 80 4.73 x 10' 294 90 7.01 x 104 418 95 8.59 x 10' 505 100 1.01 x 105 598 120 1.99 x 105 1095 150 4.76 x 10' 2430 200 1.55 x 106 7090 220 2.32 x 10° 10200
Q: Part A What is the speed of a proton after being accelerated from rest through a 4.4x107 V potential…
A: Kinetic energy of a charge particle accelerated by a potential difference V = q*V q of proton = 1.6…
Q: True or False. As the charge in the capacitor increases, the voltage AV_R across the resistor…
A: The question is asking whether the voltage across a resistor decreases as the charge in a capacitor…
Q: This is not a exam problem I need help on this homework question. I woul like help on a step by step…
A: The objective of the question is to find the power required to drive the heat pump and to determine…
Q: A conducting wire shaped in a "U shape" is connected to a 5 ohm light bulb. The 0.8 T external…
A: The objective of the question is to calculate the induced electromotive force in a conducting wire…
Q: View Policies Current Attempt in Progress A concave mirror has a focal length of 31.8 cm. The…
A: The objective of this question is to find the object and image distances for a concave mirror given…
Q: A small block on a frictionless, horizontal surface has a mass of 2.50×10−2 kg. It is attached to a…
A: Step 1:Mass is 2.5×10−2kgRadial distance r1 is 0.3mRadial distance r1 is 0.15mAngular speed ω1 is…
Q: Suppose a 175 kg motorcycle is heading toward a hill at a speed of 34 m/s. The two wheels weigh 12…
A: The objective of the question is to find out how high the motorcycle can coast up the hill without…
Q: 5. Two waves of the same amplitude and wavelength A meet. They interfere destructively. What is a…
A: To interfere destructively, one wave's crest must overlap with other wave's trough. And from the…
Q: Two blocks connected by a string are pulled across a horizontal surface by a force applied to one of…
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: An object is 21 cm in front of a diverging lens that has a focal length of -9.6 cm. How far in front…
A:
Q: Suppose 1.10 × 10^3 moles of a monatomic ideal gas undergoes an isothermal expansion as shown in the…
A:
Q: Refer to the drawing that accompanies Check Your Understanding Question 14. Suppose that the voltage…
A: Take note:v = speed/velocityV = voltage (emf)B = magnetic fieldl = length Condition: magnetic field…
Q: In the figure here, a small, solid, uniform ball is to be shot from point P so that it rolls…
A: 1. Analyze the hinge and force application point:From the image, identify the hinge point (the point…
Q: What is the thinnest soap film (excluding the case of zero thickness) that appears black when…
A: Using the concept of destructive interference, we want the two reflected rays to interact…
Q: Calculate or for spherical potential well of radius b and depth Vo (for high energy particle…
A: Here's the solution for calculating the scattering cross-section (σ) for a spherical potential well…
Q: Three point masses are arranged in a triangular pattern, as shown below. The massvalues are m1 = 125…
A: Solution:Step 1: Calculate individual gravitational forces.We can use Newton's law of universal…
Q: What would be the height of the atmosphere if the air density (a) were uniform and (b) decreased…
A: note that at sea level, y = 0, p = p0 = 1 atm at the topmost layer of atomsphere, y=h, p =…
Q: Consider an airplane with a total wing surface of 100 square meters. At a certain speed the…
A: Given: The total surface area is 100 m2 and the difference in the air pressure is 4% of atmospheric…
Q: Figure m 1 of 1 Part A Calculate the angular momentum of a particle of mass m moving with constant…
A:
Q: Maxwell Relation
A:
Q: The horizontal surface on which the objects slide is frictionless. If M = 1.0 kg and F = 17.7 N,…
A: The objective of the question is to find the force exerted by the small block on the large block.…
Q: A car of mass 1670kg initially at a speed of 80 km/m crushes into a wall, if the accident lasted for…
A:
Q: A perfectly reflecting flat mirror is uniformly illuminated by light striking it perpendicularly.…
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: Don't provide hand writing solution
A: The objective of the question is to find the angular speed of the rod and point particle when it has…
Q: A current of strength I=1A flows through the copper wire. The wire consists of two straight parts…
A: The objective of the question is to find the direction of the magnetic field when the total force on…
Q: Please have step by step solution and explain
A: Step 1: Step 2: Step 3: Step 4:
Q: Write a Java method isLinkedListPalindrome that takes the head of a singly linked list as input and…
A: To determine if a singly linked list is a palindrome, we need to compare elements from the beginning…
Q: Find the net electric field intensity at point C in the diagram below (just in case it’s hard to…
A: Please see the attached image for the solution.If there are queries, please do not hesitate to ask.…
Q: 3.27 A metal rod is bent into a circular arc of radius R with an arc of 270º. One end of the rod is…
A: Step 1:Step 2: Step 3: Step 4:
Q: Shown to the right is a block of mass m = 11.4 kg sitting on a ramp that makes an angle θ = 27∘ with…
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: A simple pendulum is made of a small blob of mass m = 3.000 kg attached to the end of an…
A: Step 1:Step 2:Step 3:
Q: (a) Find the critical angle, 0c, for light refracting from glass (ng = 1.58) into water (n = 1.33).…
A: Approach to solving the question:Please see attached photos for detailed solutions. Thank you.…
Q: Question Eight uncharged capacitors with equal capacitances are combined in parallel. The…
A: Solution:9 To find the capacitance C' of each capacitor, we can use the formula:Q=C′∗Vwhere Q is the…
Q: A converging lens with a focal length of 90.0 cm forms an image of an object with height 3.20 cm…
A: Step 1:Step 2:
Q: A single clothesline is attached to two poles 10.0 m apart. A pulley holding a mass (11 = 294 N…
A: The scenario can be sketched as follows (figure 1) Given, Weight mg = 294 N. Distance between…
Q: Sound intensity D (decibels) is measured relative to the lowest human–detectable sound I0 = 10–12 W…
A: Lowest human detectable sound intensity, I0=10−12 W.m−2Intensity of jet, I=9.5×102 W.m−2
Q: Part A Determine the angular momentum of a 78-g particle about the origin of coordinates when the…
A: Step 1:To find the x-component of angular momentum, you typically need to know the angular momentum…
Q: A long solenoid of length 9.40 × 10-2 m and cross-sectional area 5.0 × 10-5 m² contains 6500 turns…
A: The EMF induced in the coil when the changing magnetic field passes through it. Step 1: List down…
Q: An aluminum bar moves with a speed 2m/s between two copper roads in a magnetic field of 2T as…
A: Given data:- Length of the aluminium bar is l=10mMagnetic field is B=2TVelocity of the rod is…
Q: 3. Pulling a yoyo at an angle - (Objective 27) A solid cylindrical with mass M and radius R that is…
A: Step 1: Step 2: Step 3: Step 4:
Q: A horizontal pipe has a diameter of 1.10 cm at the narrow portion and 4.50 cm at the wide part.…
A: The objective of this question is to find the water flow speeds at the wide and narrow portions of…
Q: Please solve only "e" and "f" part. Rest all parts are answerd. Expert gave only answer of first…
A: The objective of the question is to determine how the magnitude of the electric field at various…
Q: A. Two balls move as shown in the figure. (Figure 1) What is the speed of ball 1 in a referece frame…
A:
Q: The diagram shows the top half of a current carrying wire loop moving through a piece of cardboard.…
A: The right-hand rule can be used to determine the direction of the magnetic field around a…
Q: 5. A uniform external magnetic field, created by something you don't see here, points upward, in the…
A: The objective of the question is to determine the direction of the current in the wire and to draw…
Q: The intensities I of two earthquakes are compared with the Richter scale magnitudes, M. log (I2/I1)…
A:
Q: A wooden cube with edges of length 14.0 cm is floating so that 34.0% of it is under water. How much…
A: The objective of the question is to find the mass of the wooden cube that is floating in water with…
Q: Don't provide hand writing solution
A: Approach to solving the question: Detailed explanation:If you want revisions, please request for…
Q: Please help me , i need to fast solution.
A: Based on the image you sent, it appears to be a circuit containing a battery (voltage source, V),…
Q: The near point of a certain hyperopic eye is 100 cm in front of the eye. Find the focal length and…
A: As we know that the power of a lens is inverse of the focal length. ( and here f has unit of meter…
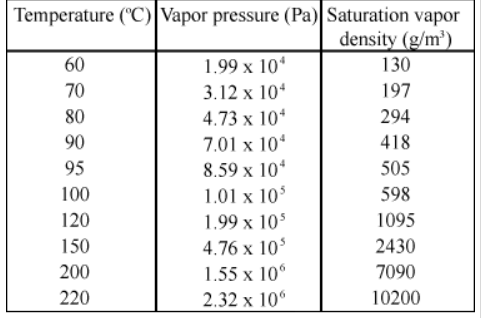
The boiling point of water can change significantly with altitude, as the following table shows.
What is the atmospheric pressure, in pascals, at the top of Mt. Everest on a day when water boils there at a temperature of 70.0°C?
Unlock instant AI solutions
Tap the button
to generate a solution
Click the button to generate
a solution
- The law of atmospheres states that the number density of molecules in the atmosphere depends on height y above sea level according to nV(y)=n0en0gy/kaT where n0 is the number density at sea level (where y = 0). The average height of a molecule in the Earths atmosphere is given by yavg=0ynV(y)dy0nV(y)dy=0yen0gy/kaTdy0en0gy/kaTdy (a) Prove that this average height is equal to kBT/m0g. (b) Evaluate the average height, assuming the temperature is 10.0C and the molecular mass is 28.9 u, both uniform throughout the atmosphere.In cold weather, you can sometimes "see" your breath. What you are seeing is a mist of small water droplets, the same as in clouds and fog. Suppose air leaves your mouth with temperature 35°C and humidity 0.035 kg/m3 and mixes with an equal amount of air at 5°C. and humidity 0.005 kg/m3 What is the relative humidity of the mixed air if its temperature and humidity equal the averages of those of the two original air masses? Represent what happens by plotting three points in a graph like Figure 5.34.17. Which of the following three statements concerning relative humidity values of 30% and 40% are true? Note that when the relative humidityis 30%, the air temperature may be diff erent than it is when the relativehumidity is 40%. A. It is possible that at a relative humidity of 30% there is a smaller partial pressure of water vapor in the air than there is at a relativehumidity of 40%.B. It is possible that there is the same partial pressure of water vapor in the air at 30% and at 40% relative humidity. C. It is possible that at a relative humidity of 30% there is a greater partial pressure of water vapor in the air than there is at a relative humidity of 40%.(a) A, B, and C (b) Only A and B (c) Only A and C (d) Only B and C(e) Only A
- 6. At an altitude of 160 km, the density of air is 1.5 * 10 ^ - 9; kg / (m ^ 3) and the temperature is approximately 500 K. What is the pressure ?31 - In a place where the pressure is 102 kPa and the temperature is 33ºC, the mouth of an empty 27-liter bottle is opened and filled with air. What is the mass of the air filled into the bottle? a) 0.0407 kg B) 0.0229 kg NS) 0.0026 kg D) 0.0019 kg TO) 0.0314 kgThe table given shows the vapor pressure of water at 20.0ºC as 2.33×103 Pa. Use the ideal gas law to calculate the density of water vapor in g /m3 that would create a partial pressure equal to this vapor pressure. Compare the result with the saturation vapor density given in the table.
- An air bubble has a volume of 1.50 cm3 when it is released by a submarine 1.00 x 102 m below the surface of a lake. What is the volume of the bubble when it reaches the surface? Assume the temperature and the number of air molecules in the bubble remain constant during its ascent.1. Variations in temperature and resulting changes in pressure are the main drivers of motion in the atmosphere. True or False 2. If a gas is held at a constant temperature but forced to occupy a smaller volume, the pressure of the gas increases. True or False 3. Air pressure is measured with an instrument called a decometer. True or False1) As the cork begins to move, what happens to the air inside the bottle? (Select all that apply.) a)The air pressure inside remains the same. b)The volume of the air inside decreases. c)The volume of the air inside remains the same. d)The volume of the air inside increases. e)The air pressure inside increases. f)The air pressure inside decreases. 2) A beachcomber finds a corked bottle containing a message. The air in the bottle is at atmospheric pressure and a temperature of 30.3°C. The cork has a cross-sectional area of 2.10 cm2. The beachcomber places the bottle over a fire, figuring that the increased pressure will push out the cork. At a temperature of 99°C the cork is ejected from the bottle. (a) What was the pressure in the bottle just before the cork left it?(b) What force of friction held the cork in place? Neglect any change in the volume of the bottle. 3) A tire contains air at a gauge pressure of 5.17 104 Pa at a temperature of 25.0°C. After nightfall, the temperature…
- An air bubble has a volume of 1.70 cm3 when it is released by a submarine 115 m below the surface of a lake. What is the volume of the bubble when it reaches the surface? Assume the temperature and the number of air molecules in the bubble remain constant during its ascent. ?cm3Air in human lungs has a temperature of 37.0ºC and a saturation vapor density of 44.0 g/m3 . (a) If 2.00 L of air is exhaled and very dry air inhaled, what is the maximum loss of water vapor by the person? (b) Calculate the partial pressure of water vapor having this density, and compare it with the vapor pressure of 6.31×103 N/m2.The temperature of the air in thermals decreases about 10°C for each 1,000 m they rise. If a thermal leaves the ground with a temperature of 35°C and a relative humidity of 77 percent, at what altitude will the air become saturated and the water vapor begin to condense to form a cloud? (In other words, at what altitude does the temperature equal the dew point?)__________ m





