The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. Write a balanced chemical equation, including physical state symbols, for the D-O decomposition of solid sodium azide (NaN) into solid sodium and gaseous dinitrogen. 2. Suppose 12.0 L of dinitrogen gas are produced by this reaction, at a temperature of 13.0 °C and pressure of exactly 1 atm. Calculate the mass of sodium azide that must have reacted. Round your answer to 3 significant digits. Dlo X
The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. Write a balanced chemical equation, including physical state symbols, for the D-O decomposition of solid sodium azide (NaN) into solid sodium and gaseous dinitrogen. 2. Suppose 12.0 L of dinitrogen gas are produced by this reaction, at a temperature of 13.0 °C and pressure of exactly 1 atm. Calculate the mass of sodium azide that must have reacted. Round your answer to 3 significant digits. Dlo X
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 32QAP: Nitrogen trifluoride gas reacts with steam to produce the gases HF, NO, and NO2. (a) Write a...
Related questions
Question
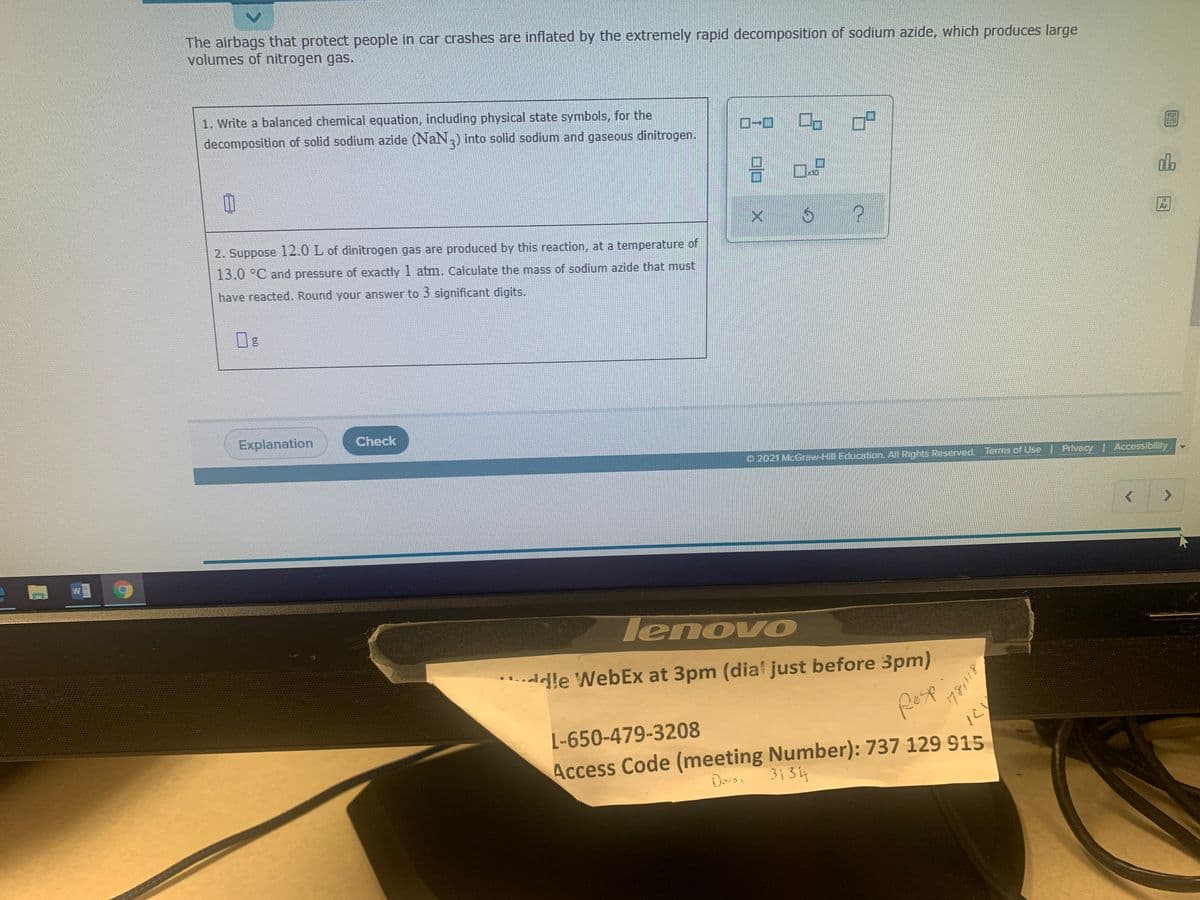
Transcribed Image Text:The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large
volumes of nitrogen gas.
1. Write a balanced chemical equation, including physical state symbols, for the
decomposition of solid sodium azide (NaN,) into solid sodium and gaseous dinitrogen.
alb
Ar
2. Suppose 12.0 L of dinitrogen gas are produced by this reaction, at a temperature of
13.0 °C and pressure of exactly 1 atm. Calculate the mass of sodium azide that must
have reacted. Round your answer to 3 significant digits.
Explanation
Check
2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessibility
<.
lenovo
dle WebEx at 3pm (dia' just before 3pm)
Resp
L-650-479-3208
Access Code (meeting Number): 737 129 915
Dois,
3134
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning