The Aufbau Principle describes the order of subshells in an atom in terms of energy. Higher numbered shells have higher energy. It is complicated by occasiona overlapping. The specific order of subshells using the Aufbau Principle can be found using the diagonal arrow diagram found in Chapter 8, and this is the same order found on the periodic table. What is the order of subshells in period 7? (there are 4) 7s a. 1s b. 2s С. 3s d. 4s е. 5s f. 6s g. 7s h. 1p i. 2p j. Зр k. 4p I. 5p m. 6p n. 7p o. 1d р. 2d q. 3d r. 4d s. 5d t. 6d u. 7d v. 1f w. 2f y. 3f z. 4f aa. 5f bb. 6f сс. 7f
The Aufbau Principle describes the order of subshells in an atom in terms of energy. Higher numbered shells have higher energy. It is complicated by occasiona overlapping. The specific order of subshells using the Aufbau Principle can be found using the diagonal arrow diagram found in Chapter 8, and this is the same order found on the periodic table. What is the order of subshells in period 7? (there are 4) 7s a. 1s b. 2s С. 3s d. 4s е. 5s f. 6s g. 7s h. 1p i. 2p j. Зр k. 4p I. 5p m. 6p n. 7p o. 1d р. 2d q. 3d r. 4d s. 5d t. 6d u. 7d v. 1f w. 2f y. 3f z. 4f aa. 5f bb. 6f сс. 7f
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter8: The Periodic Table: Structure And Trends
Section: Chapter Questions
Problem 8.90QE
Related questions
Question
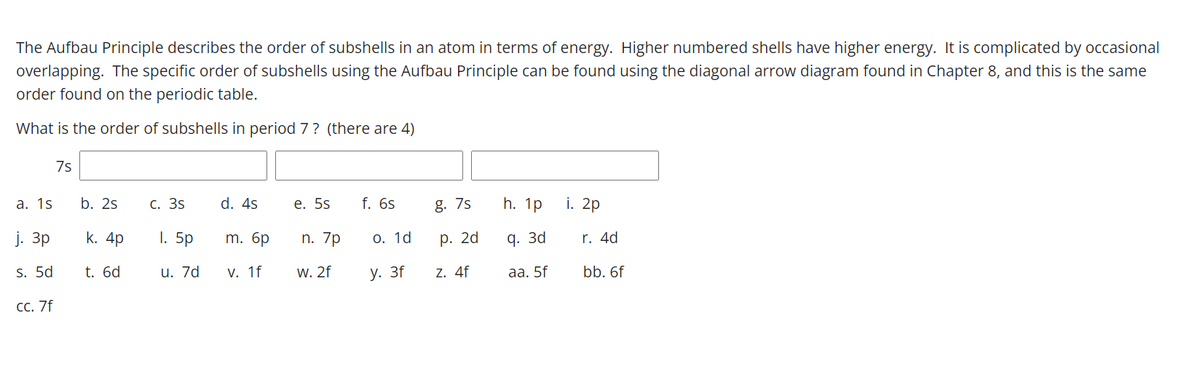
Transcribed Image Text:The Aufbau Principle describes the order of subshells in an atom in terms of energy. Higher numbered shells have higher energy. It is complicated by occasional
overlapping. The specific order of subshells using the Aufbau Principle can be found using the diagonal arrow diagram found in Chapter 8, and this is the same
order found on the periodic table.
What is the order of subshells in period 7? (there are 4)
7s
а. 1s
b. 2s
С. 3s
d. 4s
е. 5s
f. 6s
g. 7s
h. 1p
i. 2p
j. 3p
k. 4p
1. 5p
m. 6p
n. 7p
О. 1d
р. 2d
q. 3d
r. 4d
s. 5d
t. 6d
u. 7d
V. 1f
W. 2f
у. 3f
Z. 4f
aа. 5f
bb. 6f
СС. 7f
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
