The change in vapor pressure of a particular liquid at temperature T (in kelvins) is the atmospheric pressure P at which no net evaporation takes place. Use the table to estimate P' (T) for T = 303, 323, 343. T (K) 293 303 313 323 333 343 353 P (atm) 0.0394 0.0482 0.08171 0.1311 0.2111 0.3173 0.5002 Use the difference quotient approximation with h = 10. (Use decimal notation. Give your answers to five decimal places.)
The change in vapor pressure of a particular liquid at temperature T (in kelvins) is the atmospheric pressure P at which no net evaporation takes place. Use the table to estimate P' (T) for T = 303, 323, 343. T (K) 293 303 313 323 333 343 353 P (atm) 0.0394 0.0482 0.08171 0.1311 0.2111 0.3173 0.5002 Use the difference quotient approximation with h = 10. (Use decimal notation. Give your answers to five decimal places.)
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 4P
Related questions
Question
Hello, can you please help me with this calculaus homework? This homework is a science question. I have attached the question below:
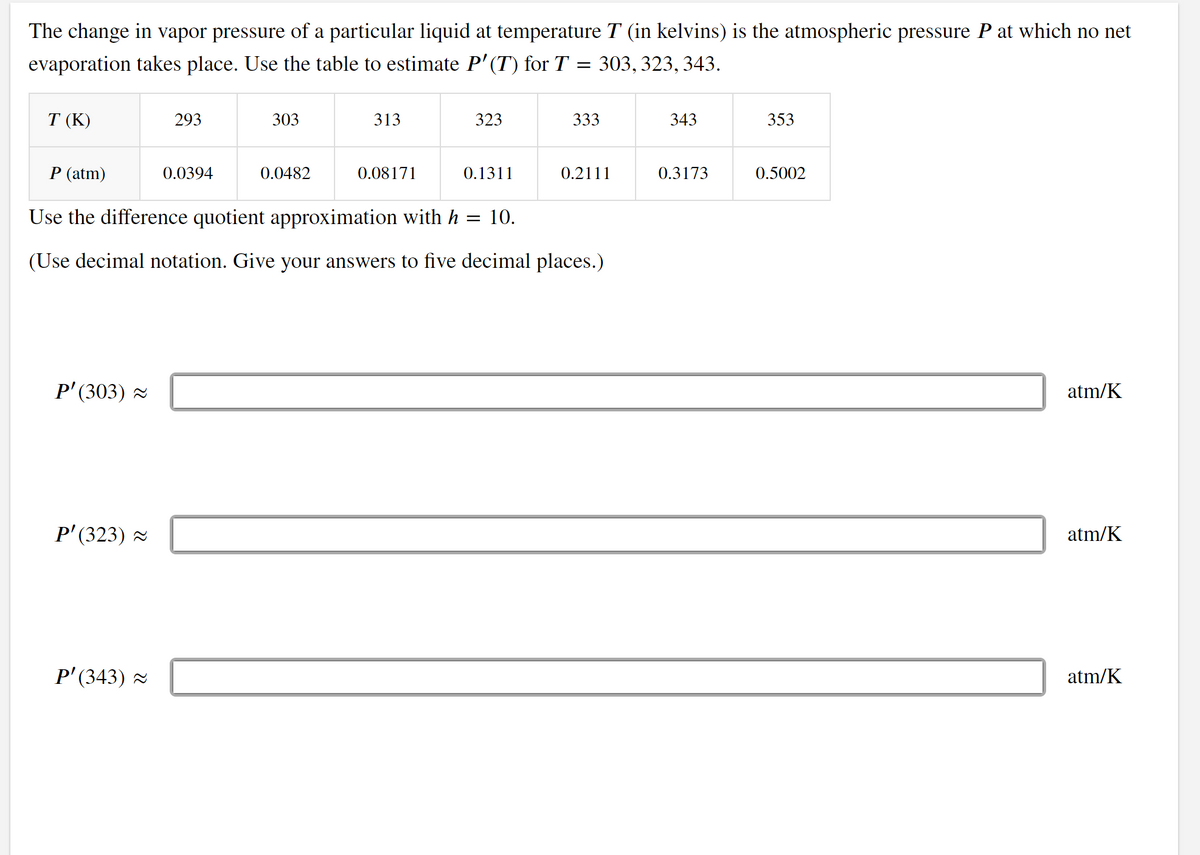
Transcribed Image Text:The change in vapor pressure of a particular liquid at temperature T (in kelvins) is the atmospheric pressure P at which no net
evaporation takes place. Use the table to estimate P'(T) for T
303, 323, 343.
T (K)
293
303
313
323
333
343
353
P (atm)
0.0394
0.0482
0.08171
0.1311
0.2111
0.3173
0.5002
Use the difference quotient approximation with h
10.
(Use decimal notation. Give your answers to five decimal places.)
P'(303) 2
atm/K
Р'(323) ~
atm/K
P' (343) 2
atm/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning