The compounds in each part below have the same (or similar) molecular weights. Which compound in cach part would you expect to have the higher boiling point? Explain your answers. (a) OH or (c) он HO HO. (b) (CH),N or N.
The compounds in each part below have the same (or similar) molecular weights. Which compound in cach part would you expect to have the higher boiling point? Explain your answers. (a) OH or (c) он HO HO. (b) (CH),N or N.
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 32AP
Related questions
Question
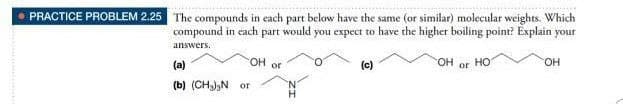
Transcribed Image Text:PRACTICE PROBLEM 2.25 The compounds in each part below have the same (or similar) molecular weights. Which
compound in each
part
would
you expect to have the higher boiling point? Éxplain your
answers.
OH or
HO.
(a)
(c)
но
OH
or
(b) (CHa),N or
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

