The decomposition of crystalline N2O5 N2O5(s)?2NO2(g)+12O(g) is an example of a reaction that is thermodynamically favored even though it absorbs heat. At 25 ?C we have the following values for the standard state enthalpy and free energy changes of the reaction: Delta H = +109.6 kJ/mol Delta Standard Gibbs Free Energy = -30.5 kJ/mol C) Calculate delta U for this reaction at 25 C.
The decomposition of crystalline N2O5 N2O5(s)?2NO2(g)+12O(g) is an example of a reaction that is thermodynamically favored even though it absorbs heat. At 25 ?C we have the following values for the standard state enthalpy and free energy changes of the reaction: Delta H = +109.6 kJ/mol Delta Standard Gibbs Free Energy = -30.5 kJ/mol C) Calculate delta U for this reaction at 25 C.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.101PAE: 10.101 Fluorine reacts with liquid water to form gaseous hydrogen fluoride and oxygen. (a) Write a...
Related questions
Question
The decomposition of crystalline N2O5
N2O5(s)?2NO2(g)+12O(g)
is an example of a reaction that is
Delta H = +109.6 kJ/mol
Delta Standard Gibbs Free Energy = -30.5 kJ/mol
C) Calculate delta U for this reaction at 25 C.
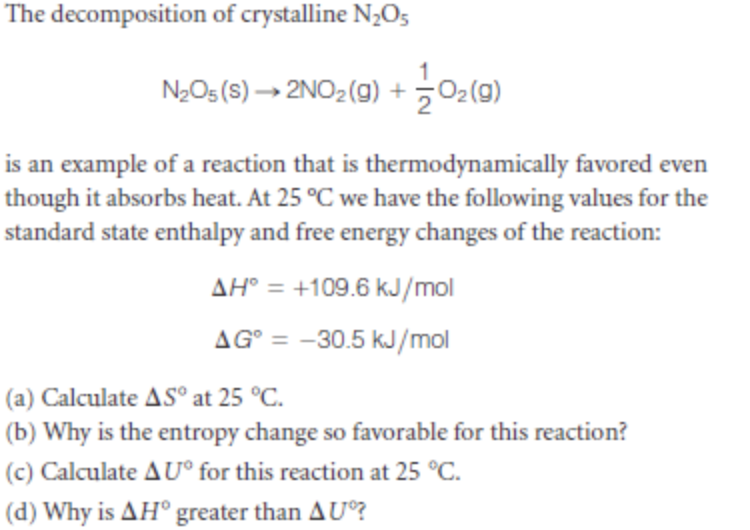
Transcribed Image Text:The decomposition of crystalline N¿O5
NeOs(S)→ 2NO2(g) + 어(미)
is an example of a reaction that is thermodynamically favored even
though it absorbs heat. At 25 °C we have the following values for the
standard state enthalpy and free energy changes of the reaction:
AH° = +109.6 kJ/mol
AG° = -30.5 kJ/mol
(a) Calculate AS° at 25 °C.
(b) Why is the entropy change so favorable for this reaction?
(c) Calculate AU° for this reaction at 25 °C.
(d) Why is AH° greater than AU?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning