The diagram above represents the photoelectric effect for a metal. When the metal surface is exposed to light with increasing frequency and energy of photons, electrons first begin to be ejected from the metal when the energy of the photons is 3.3 × 10¬19 J. 11. Which of the following is closest to the frequency of the light with photon energy of 3.3 × 10-19 J? -53 1 (A) 5.0 × 10 -1 (B) 5.0 × 10-16 -1 (C) 5.0 x 1014 (D) 5.0 x 1052 s-1 12. Color Wavelength Red 647 – 760 nm Orange 585 – 647 nm Yellow 575 – 585 nm Green 491 – 575 nm Blue 424 – 491 nm Violet 300 – 424 nm Using the wavelength information provided above, what is the color of the light?
The diagram above represents the photoelectric effect for a metal. When the metal surface is exposed to light with increasing frequency and energy of photons, electrons first begin to be ejected from the metal when the energy of the photons is 3.3 × 10¬19 J. 11. Which of the following is closest to the frequency of the light with photon energy of 3.3 × 10-19 J? -53 1 (A) 5.0 × 10 -1 (B) 5.0 × 10-16 -1 (C) 5.0 x 1014 (D) 5.0 x 1052 s-1 12. Color Wavelength Red 647 – 760 nm Orange 585 – 647 nm Yellow 575 – 585 nm Green 491 – 575 nm Blue 424 – 491 nm Violet 300 – 424 nm Using the wavelength information provided above, what is the color of the light?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter7: Electronic Structure
Section: Chapter Questions
Problem 7.30QE
Related questions
Question

Transcribed Image Text:(A) Red
(В) Orange
(C) Yellow
(D) Blue
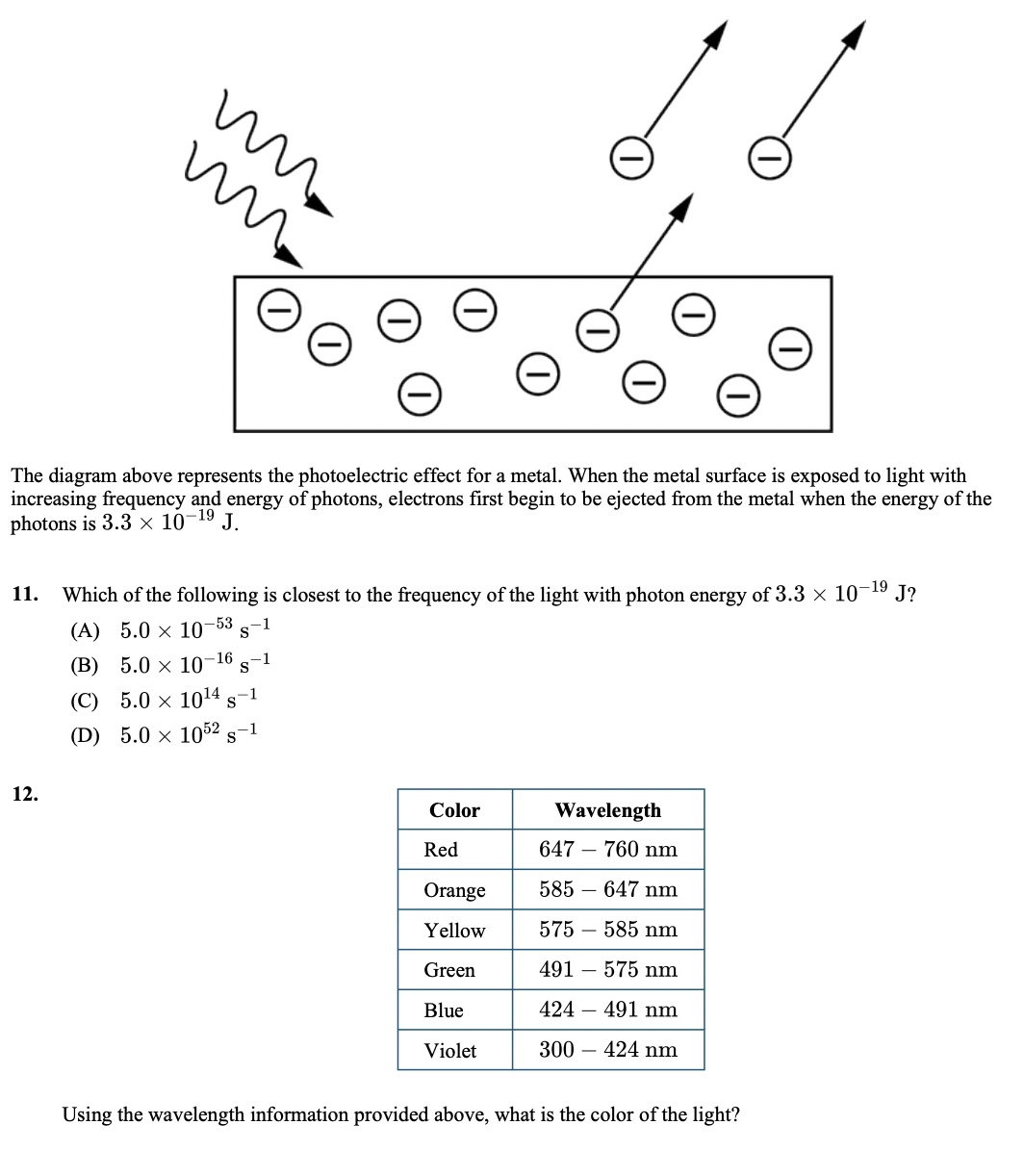
Transcribed Image Text:The diagram above represents the photoelectric effect for a metal. When the metal surface is exposed to light with
increasing frequency and energy of photons, electrons first begin to be ejected from the metal when the energy of the
photons is 3.3 × 10-19
J.
11.
Which of the following is closest to the frequency of the light with photon energy of 3.3 × 10¬19 J?
(A) 5.0 x 10-53
-1
S
-1
(В) 5.0 х 10-16
(С) 5.0 х 1014 s-1
(D) 5.0 × 1052 s-1
12.
Color
Wavelength
Red
647 – 760 nm
Orange
585 – 647 nm
Yellow
575 – 585 nm
Green
491 – 575 nm
Blue
424 – 491 nm
Violet
300 – 424 nm
Using the wavelength information provided above, what is the color of the light?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: Solve with minitab
Q: There are 37 known isotopes of iodine, but only one, 127I, is stable. Of the two iodine isotopes,…
Q: I have a client who wants to open a franchise retail operation. I would like to ask the client 8-10…
Q: On January 1, Year 1, Victor Company issued bonds with a $250,000 face value, a stated rate of…
Q: When Ni(NO3)2 is dissolved in water it forms a light green solution. When ammonia is added to the…
Q: Draw the deflection of the needle, which way it moved, the location of positive and negative…
Q: Use Green's Theorem to evaluate
t
F= √x+5y, 4x+5y)
rd
F.nds, where
C
C is the boundary of the region…
Q: Clayton Industries has the following account balances:
Current assets
Noncurrent assets
$ 22,000…
Q: Doyle Company issued $360,000 of 10-year, 7 percent bonds on January 1, Year 2. The bonds were…
Q: On January 1 Year 1, Gordon Corporation issued bonds with a face value of $70,000, a stated rate of…
Q: Please give the appropriate product(s) for the following Diels-Alder reaction.
E
F
B
Q: I have a new client wanting to purchase a franchise retail operation. What would be 8-10 questions…
Q: A chemist fills a reaction vessel with 0.767 M lead (II) (Pb2+) aqueous solution, 0.207 M bromide…
Q: Find the recursion formula for the coefficients of the power series. For n = 10, what is the factor…
Q: Is the input wave form F(t) even or odd?
What is the frequency of the forcing function? Round to the…
Q: The temperature, H, in degrees Celsius, of a cup of coffee placed on the kitchen counter is given by…
Q: This question is over numerical methods using the following three methods shown in the problem to…
Q: https://www.apa.org/monitor/2023/11/incorporating-religion-spirituality-therapy
Does this source…
Q: Assume in each problem that the base metal is A36 steel and that the electrodes are E70XX.
10.9…
Q: 10.1 Determine the allowable load P permitted for this double shear joint connection assuming A36…
Q: AMPAD™
A
67° 17'
B
C
D
THE FOUR SIDED CLOSED TRAVERSE
HAS THE FOLLOWING ANGLES &
DISTAN CES
A= 51°…