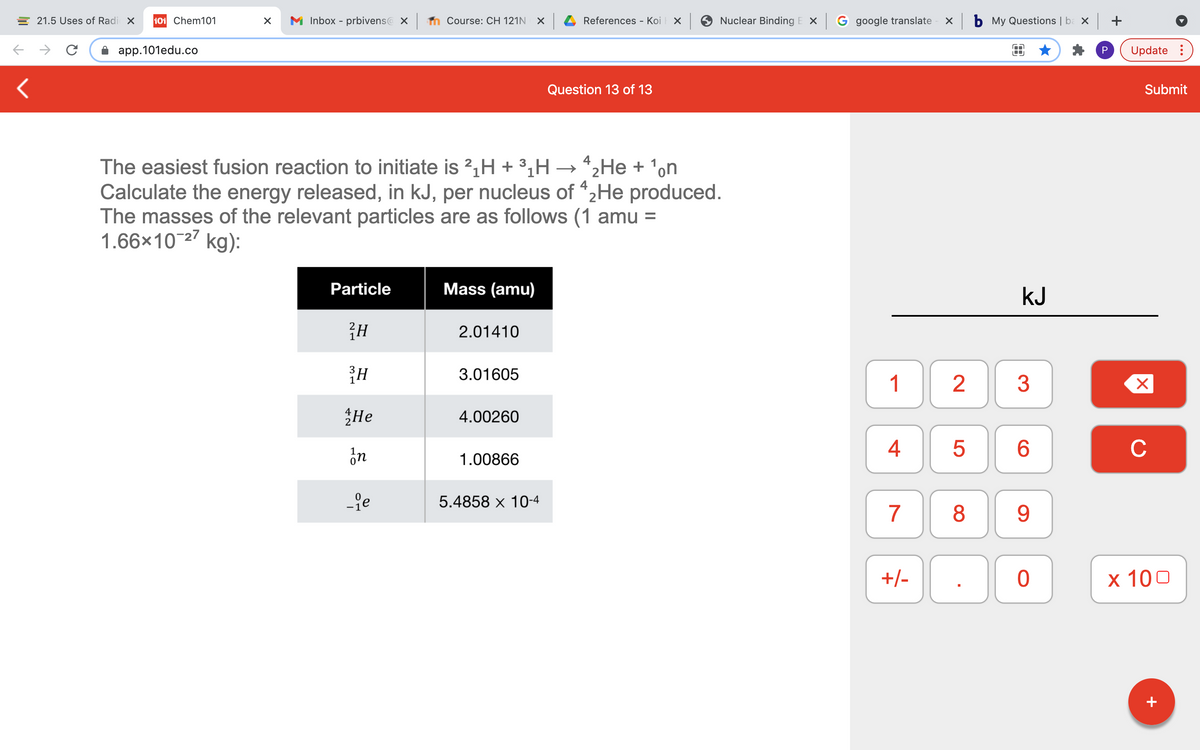
The easiest fusion reaction to initiate is 2,H + 3,H → *2He + 'on Calculate the energy released, in kJ, per nucleus of “2He produced. The masses of the relevant particles are as follows (1 amu = 1.66x10-27 kg): Particle Mass (amu) 2.01410 3.01605 He 4.00260 1.00866 -fe 5.4858 x 10-4
The easiest fusion reaction to initiate is 2,H + 3,H → *2He + 'on Calculate the energy released, in kJ, per nucleus of “2He produced. The masses of the relevant particles are as follows (1 amu = 1.66x10-27 kg): Particle Mass (amu) 2.01410 3.01605 He 4.00260 1.00866 -fe 5.4858 x 10-4
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter9: Nuclear Chemistry
Section: Chapter Questions
Problem 9.45P
Related questions
Question
100%
Where do I even begin to solve this problem ?
Transcribed Image Text:E 21.5 Uses of Radi x
101 Chem101
M Inbox - prbivens@ X
n Course: CH 121N X
References - Koi
Nuclear Binding E X
G google translate
X b My Questions | ba × +
app.101edu.co
P
Update :
Question 13 of 13
Submit
The easiest fusion reaction to initiate is 2,H + 3¿H → *2He + 'on
Calculate the energy released, in kJ, per nucleus of "2He produced.
The masses of the relevant particles are as follows (1 amu =
1.66×10-27 kg):
Particle
Mass (amu)
kJ
2.01410
3.01605
1
2
3
He
4.00260
ón
6
C
1.00866
-je
5.4858 x 10-4
7
8
9
+/-
х 100
+
4+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning