the electron ionization mass spectrum of a small neutral molecule shows a base peak at 27 and a smaller peak at 26 with a relative abundance of 20%. Deduce the structure of the unknown compound. Draw a cation that corresponds to this peak.
the electron ionization mass spectrum of a small neutral molecule shows a base peak at 27 and a smaller peak at 26 with a relative abundance of 20%. Deduce the structure of the unknown compound. Draw a cation that corresponds to this peak.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.25P
Related questions
Question
the electron ionization mass spectrum of a small neutral molecule shows a base peak at 27 and a smaller peak at 26 with a relative abundance of 20%. Deduce the structure of the unknown compound.
Draw a cation that corresponds to this peak.
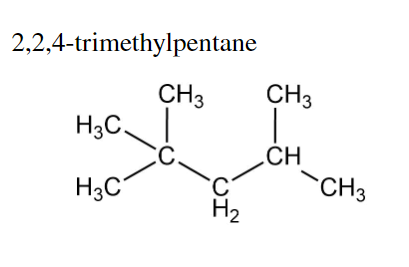
Transcribed Image Text:2,2,4-trimethylpentane
CH3
CH3
H3C
H3C
C
C'
H₂
CH
CH3
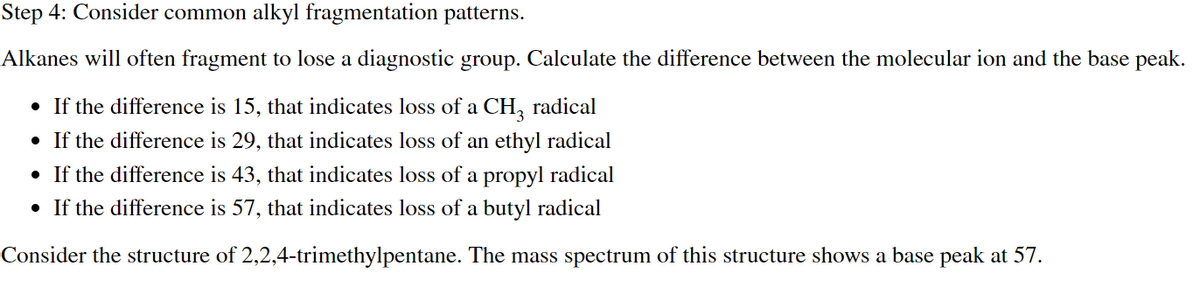
Transcribed Image Text:Step 4: Consider common alkyl fragmentation patterns.
Alkanes will often fragment to lose a diagnostic group. Calculate the difference between the molecular ion and the base peak.
• If the difference is 15, that indicates loss of a CH3 radical
• If the difference is 29, that indicates loss of an ethyl radical
• If the difference is 43, that indicates loss of a propyl radical
• If the difference is 57, that indicates loss of a butyl radical
Consider the structure of 2,2,4-trimethylpentane. The mass spectrum of this structure shows a base peak at 57.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning