The element E reacts with F to form an ionic compound EF. The element forms a solid ionic hydride while the hydride of F is gaseous at room temperature. To what group in the periodic table could E and F belong? Give reasons for your answer
The element E reacts with F to form an ionic compound EF. The element forms a solid ionic hydride while the hydride of F is gaseous at room temperature. To what group in the periodic table could E and F belong? Give reasons for your answer
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 92QRT
Related questions
Question
- The element E reacts with F to form an ionic compound EF. The element forms a solid ionic hydride while the hydride of F is gaseous at room temperature. To what group in the periodic table could E and F belong? Give reasons for your answer
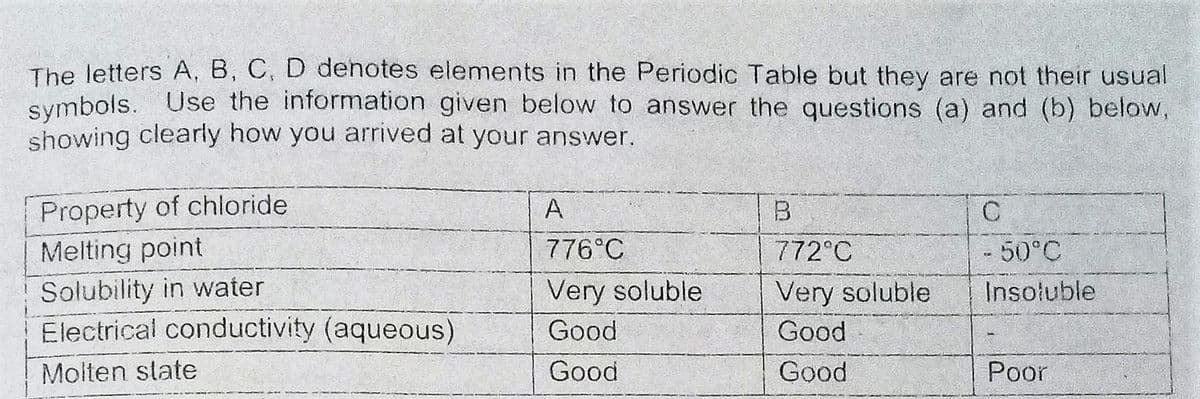
Transcribed Image Text:The letters A, B, C, D dehotes elements in the Periodic Table but they are not their usual
symbols. Use the information given below to answer the questions (a) and (b) below,
showing clearly how you arrived at your answer.
Property of chloride
Melting point
A
B
776°C
772°C
-50°C
Solubility in water
Electrical conductivity (aqueous)
Very soluble
Very soluble
Insoluble
Good
Good
Molten state
Good
Good
Poor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax