The energy of light photons varies with wavelength. Calculate the energy per mole of photons for each of the given colors of visible light. kJ red light, A = 727 nm mol green light, A = 537 nm E = kJ mol blue light, A = 407 nm E = kJ mol Splitting liquid water into hydrogen and oxygen requires an input of 286 kJ/mol. 286 kJ + H,O(1) → H, (g) + 02(g) Assuming a mechanism existed in which one photon of light could split one molecule of water, which color of visible light has enough energy to carry out this reaction? red green blue
The energy of light photons varies with wavelength. Calculate the energy per mole of photons for each of the given colors of visible light. kJ red light, A = 727 nm mol green light, A = 537 nm E = kJ mol blue light, A = 407 nm E = kJ mol Splitting liquid water into hydrogen and oxygen requires an input of 286 kJ/mol. 286 kJ + H,O(1) → H, (g) + 02(g) Assuming a mechanism existed in which one photon of light could split one molecule of water, which color of visible light has enough energy to carry out this reaction? red green blue
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section6.7: One More Electron Property: Electron Spin
Problem 1.2ACP: Calculate the energy per mole of photons (in kJ/mol) for red light with a wavelength of 700 nm....
Related questions
Question
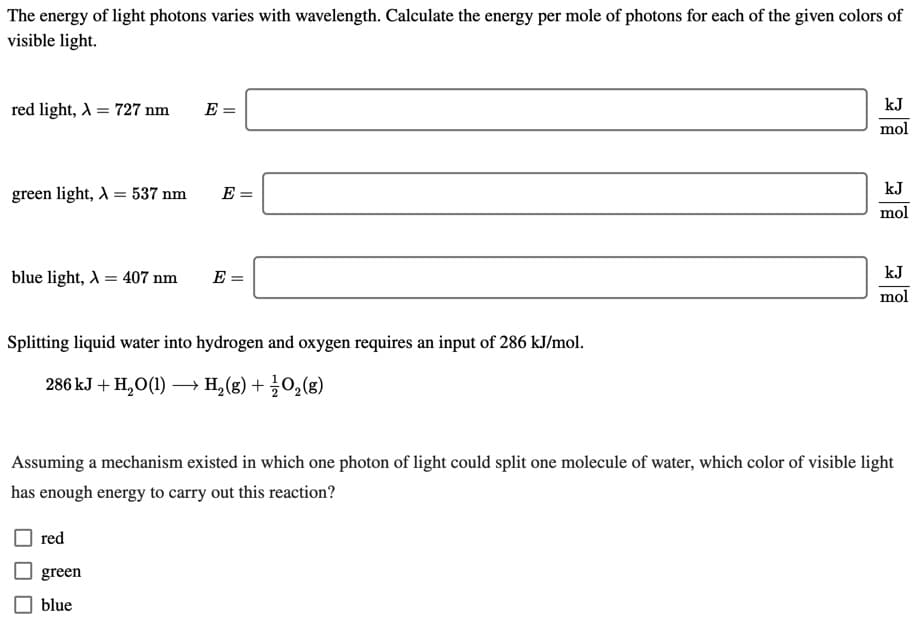
Transcribed Image Text:The energy of light photons varies with wavelength. Calculate the energy per mole of photons for each of the given colors of
visible light.
kJ
red light, A = 727 nm
mol
green light, A = 537 nm
E =
kJ
mol
blue light, A = 407 nm
E =
kJ
mol
Splitting liquid water into hydrogen and oxygen requires an input of 286 kJ/mol.
286 kJ + H,O(1) → H, (g) + 02(g)
Assuming a mechanism existed in which one photon of light could split one molecule of water, which color of visible light
has enough energy to carry out this reaction?
red
green
blue
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning