The equivalent conductance of a solution is a measure of its ability to conduct electricity. NaOH Solution Molecular Equivalent (0.01 M at 25 °C) Mass Conductance HF NaOH 39.997 238 HF 20.006 96 КОН HCl 36.461 412 HCl КОН 56.105 228 Based on the data in the table above, which of the acids or bases listed is the CLEAR ALL strongest electrolyte?
The equivalent conductance of a solution is a measure of its ability to conduct electricity. NaOH Solution Molecular Equivalent (0.01 M at 25 °C) Mass Conductance HF NaOH 39.997 238 HF 20.006 96 КОН HCl 36.461 412 HCl КОН 56.105 228 Based on the data in the table above, which of the acids or bases listed is the CLEAR ALL strongest electrolyte?
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 21E
Related questions
Question
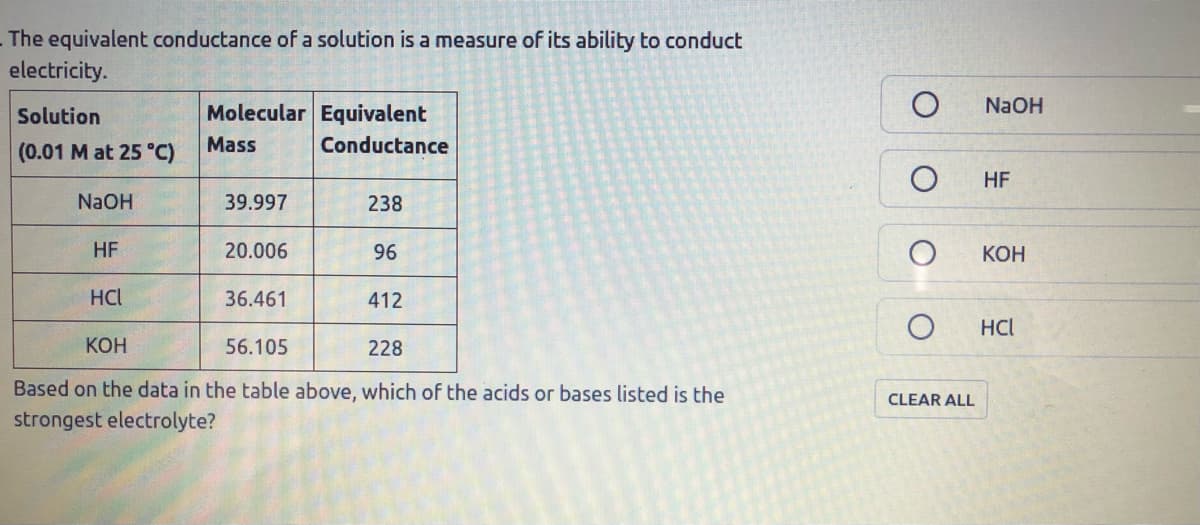
Transcribed Image Text:The equivalent conductance of a solution is a measure of its ability to conduct
electricity.
Solution
Molecular Equivalent
NaOH
(0.01 M at 25 °C)
Mass
Conductance
HF
NaOH
39.997
238
HF
20.006
96
КОН
HCl
36.461
412
HCl
КОН
56.105
228
Based on the data in the table above, which of the acids or bases listed is the
strongest electrolyte?
CLEAR ALL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning