The favorite nuclide used in positron emission tomography (PET scan) to follow glucose metabolism is fluorine-18, which decays by positron emission to oxygen-18 and has a half-life of 110 minutes. Fluorine-18 is administered in the form of fludeoxyglucose F-18; the product of this molecule's decay is glucose. HO HO, H H H H H > OH H ÓH OH НО H НО 18F 18ÓH Fludeoxyglucose F-18 A positron D-Glucose Draw the alternative chair conformations for fludeoxyglucose F-18 and select the more stable of the two.
The favorite nuclide used in positron emission tomography (PET scan) to follow glucose metabolism is fluorine-18, which decays by positron emission to oxygen-18 and has a half-life of 110 minutes. Fluorine-18 is administered in the form of fludeoxyglucose F-18; the product of this molecule's decay is glucose. HO HO, H H H H H > OH H ÓH OH НО H НО 18F 18ÓH Fludeoxyglucose F-18 A positron D-Glucose Draw the alternative chair conformations for fludeoxyglucose F-18 and select the more stable of the two.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 46E: Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a...
Related questions
Question
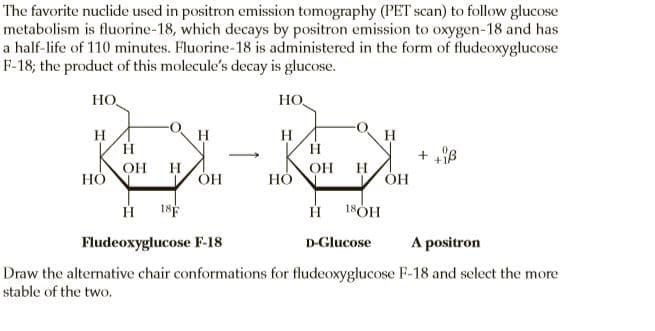
Transcribed Image Text:The favorite nuclide used in positron emission tomography (PET scan) to follow glucose
metabolism is fluorine-18, which decays by positron emission to oxygen-18 and has
a half-life of 110 minutes. Fluorine-18 is administered in the form of fludeoxyglucose
F-18; the product of this molecule's decay is glucose.
HO
HO,
H
H
H
H
H
>
OH
H
ÓH
OH
НО
H
НО
18F
18ÓH
Fludeoxyglucose F-18
A positron
D-Glucose
Draw the alternative chair conformations for fludeoxyglucose F-18 and select the more
stable of the two.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning