The following are data for the adsorption of CO on wood charcoal at 0°C. The following pressure P is in mm Hg while x is the volume of gas in cc, measured at standard conditions, adsorbed by 2.964 g of charcoal: 73 7.5 540 38.1 180 16.5 882 52.3 309 25.1 a. Find k andn of Freundlich equation b. Find graphically the constants a and b in the Langmuir equation which fit the data given c. From the results of the preceding problem, calculate the volume of CO at standard conditions adsorbed by 1 g of wood charcoal at 0°C when partial pressure of CO is 400 mm.
The following are data for the adsorption of CO on wood charcoal at 0°C. The following pressure P is in mm Hg while x is the volume of gas in cc, measured at standard conditions, adsorbed by 2.964 g of charcoal: 73 7.5 540 38.1 180 16.5 882 52.3 309 25.1 a. Find k andn of Freundlich equation b. Find graphically the constants a and b in the Langmuir equation which fit the data given c. From the results of the preceding problem, calculate the volume of CO at standard conditions adsorbed by 1 g of wood charcoal at 0°C when partial pressure of CO is 400 mm.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.13QAP
Related questions
Question
send complete solution for letter c. all of the given and details are here
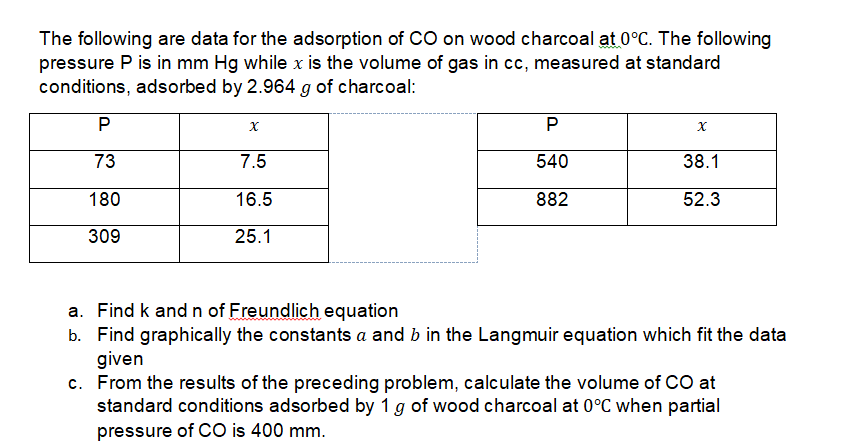
Transcribed Image Text:The following are data for the adsorption of CO on wood charcoal at 0°C. The following
pressure P is in mm Hg while x is the volume of gas in cc, measured at standard
conditions, adsorbed by 2.964 g of charcoal:
P
73
7.5
540
38.1
180
16.5
882
52.3
309
25.1
a. Find k andn of Freundlich equation
b. Find graphically the constants a and b in the Langmuir equation which fit the data
given
c. From the results of the preceding problem, calculate the volume of CO at
standard conditions adsorbed by 1 g of wood charcoal at 0°C when partial
pressure of CO is 400 mm.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning