The following compound is a carboxylic acid that contains a bromine atom: C4H,02Br. The peak at 10.97 ppm was moved on to the chart (which runs only from 0 to 8 ppm) for clarity. What is the structure of the compound? H NMR 60 MHz Crtegral1 Integre1 Intergral2 Use the editor to format your answer
The following compound is a carboxylic acid that contains a bromine atom: C4H,02Br. The peak at 10.97 ppm was moved on to the chart (which runs only from 0 to 8 ppm) for clarity. What is the structure of the compound? H NMR 60 MHz Crtegral1 Integre1 Intergral2 Use the editor to format your answer
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.8: More Complex Spin–spin Splitting Patterns
Problem 15P: 3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled...
Related questions
Question
the quation is in the photo
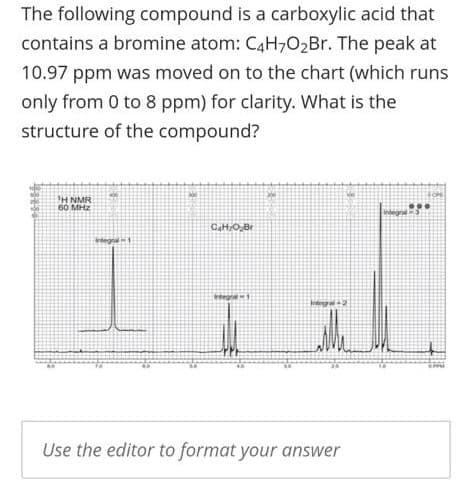
Transcribed Image Text:The following compound is a carboxylic acid that
contains a bromine atom: C4H,02Br. The peak at
10.97 ppm was moved on to the chart (which runs
only from 0 to 8 ppm) for clarity. What is the
structure of the compound?
H NMR
60 MHz
CaHO,Br
Crtegral1
tntegre1
Use the editor to format your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

