The following ingredients are thrown into an insulated cup. Find the equilibrium temperature of the mixture. There are no phase changes. (kg` Volume (m³) Density \m³ Specific Heat () Initial Temperature (K) Name \kg K, Water 1 1000 4181 273 Gold 0.1 19,300 10,490 4500 130 200 Silver 0.05 235 550 Titanium 0.5 527 783 Nickle 0.15 8890 443 600 Brass 0.1 8500 385 780
The following ingredients are thrown into an insulated cup. Find the equilibrium temperature of the mixture. There are no phase changes. (kg` Volume (m³) Density \m³ Specific Heat () Initial Temperature (K) Name \kg K, Water 1 1000 4181 273 Gold 0.1 19,300 10,490 4500 130 200 Silver 0.05 235 550 Titanium 0.5 527 783 Nickle 0.15 8890 443 600 Brass 0.1 8500 385 780
Related questions
Question
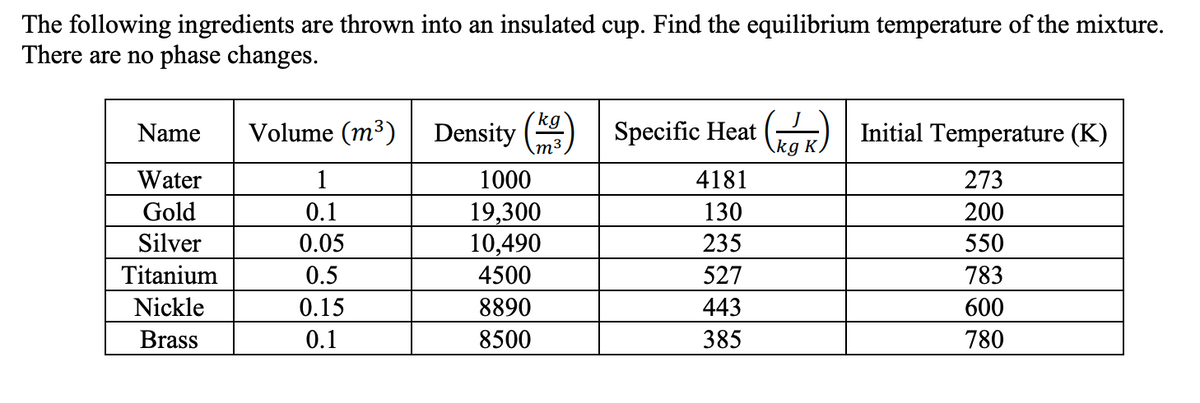
Transcribed Image Text:The following ingredients are thrown into an insulated cup. Find the equilibrium temperature of the mixture.
There are no phase changes.
(kg
Volume (m³)
Density
m³
Specific Heat
kg
Gar) | Initial Temperature (K)
Name
Water
1
1000
4181
273
19,300
10,490
Gold
0.1
130
200
Silver
0.05
235
550
Titanium
0.5
4500
527
783
Nickle
0.15
8890
443
600
Brass
0.1
8500
385
780
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps
