The following questions refer to figures with circles and ovals. The clear circles represent water molecules, and the filled ovals represent sugar molecules. The dashed line represents a semi-permeable membrane, which is impermeable to sugars, but permeable to water. Answer the questions based on Fig. 1 figures 1 & 2 below. 1. Looking at Fig. 1, count the numbers of water circles and sugar circles, and calculate the % concentration of sugar in compartments A and B % concentration sugar in A % concentration sugar in B 2. Movement of molecules will occur in both directions, but in which direction will the net movement of water be greater? 3. As a consequence of the movement of water, what will happen to the volumes of the two compartments in Fig. 1? 4. Looking at Fig. 2, will osmosis occur more rapidly in A or B? Why? Fig. 2 B
The following questions refer to figures with circles and ovals. The clear circles represent water molecules, and the filled ovals represent sugar molecules. The dashed line represents a semi-permeable membrane, which is impermeable to sugars, but permeable to water. Answer the questions based on Fig. 1 figures 1 & 2 below. 1. Looking at Fig. 1, count the numbers of water circles and sugar circles, and calculate the % concentration of sugar in compartments A and B % concentration sugar in A % concentration sugar in B 2. Movement of molecules will occur in both directions, but in which direction will the net movement of water be greater? 3. As a consequence of the movement of water, what will happen to the volumes of the two compartments in Fig. 1? 4. Looking at Fig. 2, will osmosis occur more rapidly in A or B? Why? Fig. 2 B
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 106SCQ
Related questions
Question
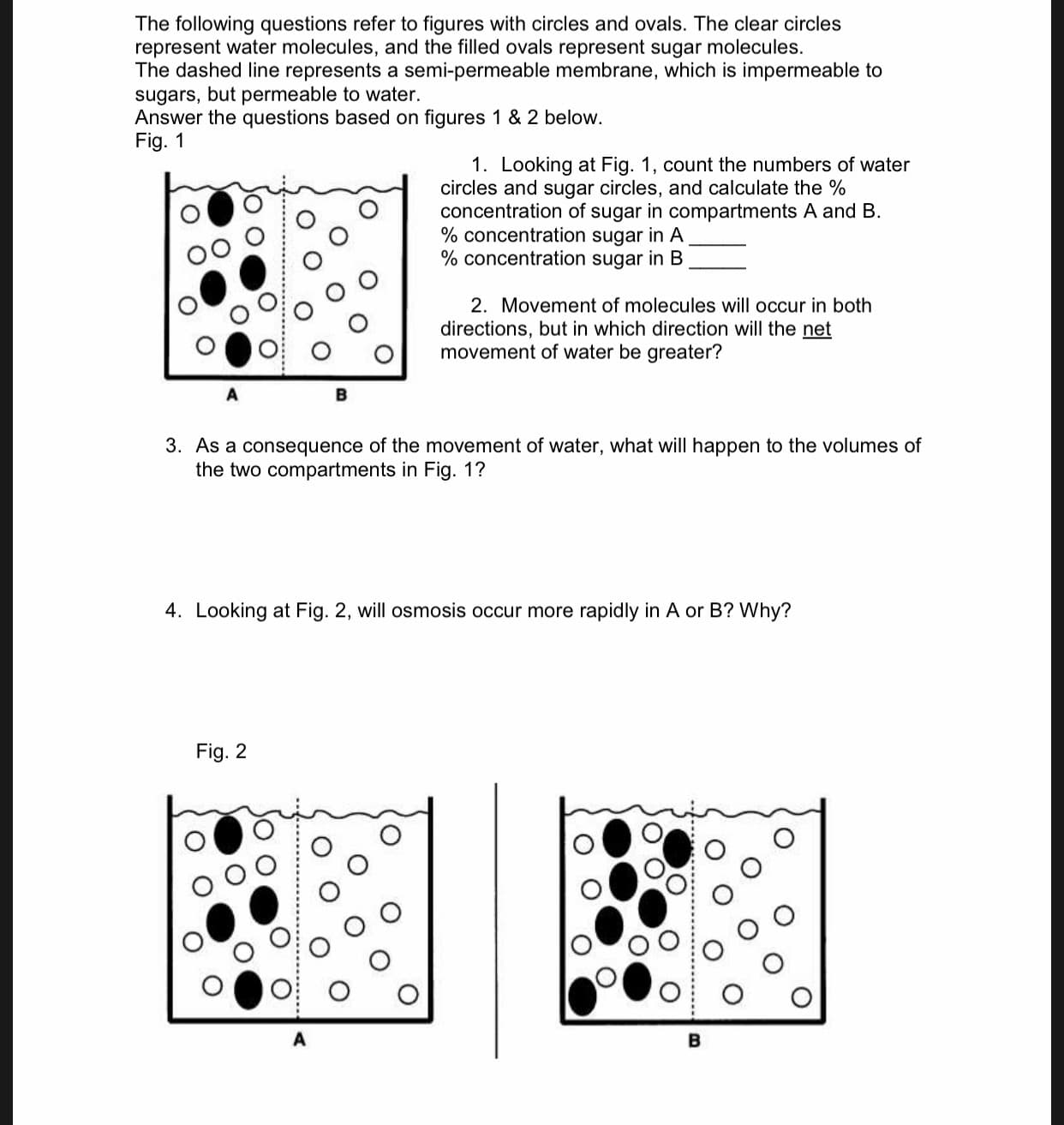
Transcribed Image Text:The following questions refer to figures with circles and ovals. The clear circles
represent water molecules, and the filled ovals represent sugar molecules.
The dashed line represents a semi-permeable membrane, which is impermeable to
sugars, but permeable to water.
Answer the questions based on
Fig. 1
figures 1 & 2 below.
1. Looking at Fig. 1, count the numbers of water
circles and sugar circles, and calculate the %
concentration of sugar in compartments A and B
% concentration sugar in A
% concentration sugar in B
2. Movement of molecules will occur in both
directions, but in which direction will the net
movement of water be greater?
3. As a consequence of the movement of water, what will happen to the volumes of
the two compartments in Fig. 1?
4. Looking at Fig. 2, will osmosis occur more rapidly in A or B? Why?
Fig. 2
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning