The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balanced REDUCTION half reaction. NH,OH + S,0,-–→N,H4 + SO;?- Reactants Products
The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balanced REDUCTION half reaction. NH,OH + S,0,-–→N,H4 + SO;?- Reactants Products
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 20E: An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed...
Related questions
Question
4
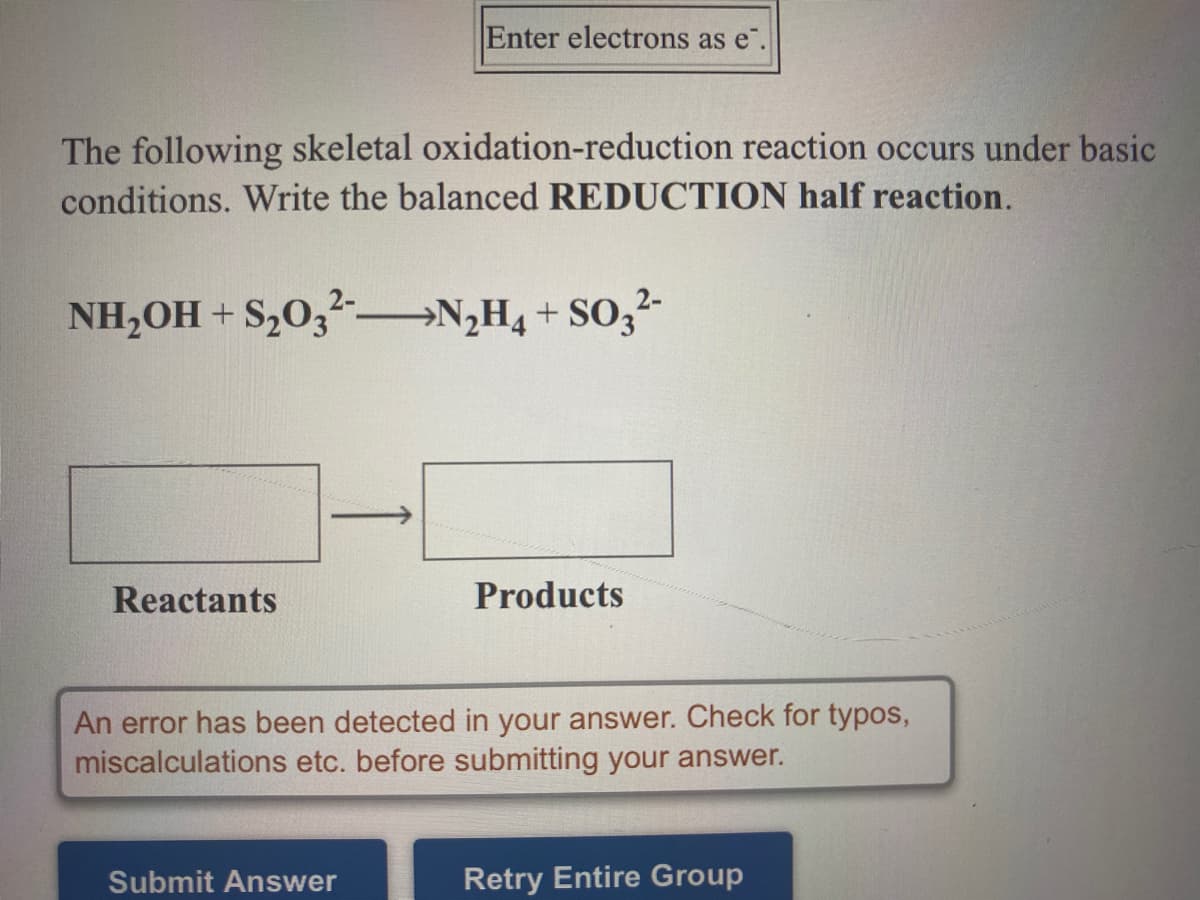
Transcribed Image Text:Enter electrons as e".
The following skeletal oxidation-reduction reaction occurs under basic
conditions. Write the balanced REDUCTION half reaction.
NH,OH + S,0,-
→N;H4 + SO,2-
Reactants
Products
An error has been detected in your answer. Check for typos,
miscalculations etc. before submitting your answer.
Submit Answer
Retry Entire Group
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
