The following table shows data from the titration of four 10.0 mL samples of a solution containing hydrochloric acid, HCI(aq), using a 0.231 mol/L sodium hydroxide standard solution. Bromothymol blue is used as an indicator to detect when the endpoint is reached. Titration of 10.0 mL HCl(aq) with 0.231 mol/L NaOH(aq) Trial #1 # 2 #3 # 4 final burette reading (mL) 17.9 35.0 22.9 40.1 initial burette reading (mL) 0.3 17.9 5.9 22.9 volume of NaOH(aq) added (mL) colour at endpoint blue green green green a) Select the most consistent trials and calculate the average volume of titrant added. b) Calculate the concentration of the hydrochloric acid solution.
The following table shows data from the titration of four 10.0 mL samples of a solution containing hydrochloric acid, HCI(aq), using a 0.231 mol/L sodium hydroxide standard solution. Bromothymol blue is used as an indicator to detect when the endpoint is reached. Titration of 10.0 mL HCl(aq) with 0.231 mol/L NaOH(aq) Trial #1 # 2 #3 # 4 final burette reading (mL) 17.9 35.0 22.9 40.1 initial burette reading (mL) 0.3 17.9 5.9 22.9 volume of NaOH(aq) added (mL) colour at endpoint blue green green green a) Select the most consistent trials and calculate the average volume of titrant added. b) Calculate the concentration of the hydrochloric acid solution.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.102QE
Related questions
Question
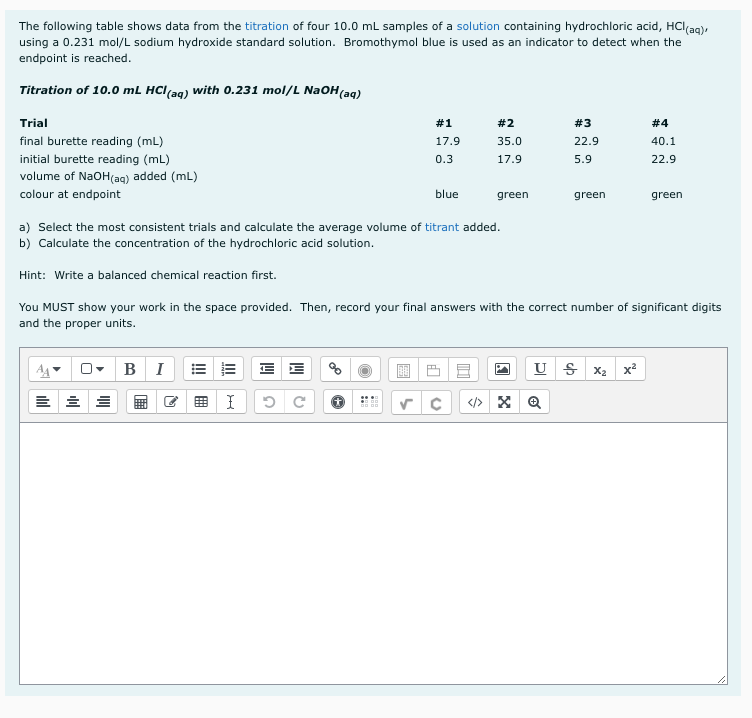
Transcribed Image Text:The following table shows data from the titration of four 10.0 mL samples of a solution containing hydrochloric acid, HClag),
using a 0.231 mol/L sodium hydroxide standard solution. Bromothymol blue is used as an indicator to detect when the
endpoint is reached.
Titration of 10.0 mL HCl(aq) with 0.231 mol/L NaOH(aq)
Trial
#1
#2
#3
# 4
final burette reading (mL)
17.9
35.0
22.9
40.1
initial burette reading (mL)
0.3
17.9
5.9
22.9
volume of NaOH(aq) added (mL)
colour at endpoint
blue
green
green
green
a) Select the most consistent trials and calculate the average volume of titrant added.
b) Calculate the concentration of the hydrochloric acid solution.
Hint: Write a balanced chemical reaction first.
You MUST show your work in the space provided. Then, record your final answers with the correct number of significant digits
and the proper units.
B I
E E
of
U S x2 x²
田I
</> X
!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
