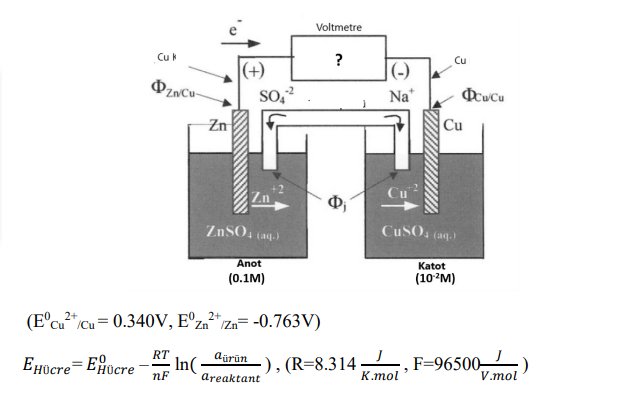
The galvanic cell test setup given in the figure is at 25 ° C. It Show the total reaction by writing the anodic and cathodic reactions of the galvanic cell. The potential to be read by the voltmeter connected to the system and the Nernst cell potential calculate.
Q: An engineer is assigned to design an electrochemical cell that will deliver a potential of exactly…
A: In this we have design an electrochemical cell of given electrode potential i.e 1.52 V with…
Q: An electrolytic cell contains inert electrodes in a solution of acidified copper(II) nitrate. Write…
A: Two platinum inert electrodes are dipped in Cu(NO3)2 aqueous solution. One electrode is connected to…
Q: Give the condition for Daniell Cell in which there is no flow of electrons or current or we can…
A: To find: The condition for Daniel cell in which there is no flow of electrons or current or we can…
Q: Sketch the galvanic cell based on the following overall reactions. Calculate ɛ°; show the direction…
A: Here we are asked to find out the E0 value. So considering the half reactions Fe 2+ (aq)→ Fe3+ (aq)+…
Q: Consider the following general voltaic cell:Identify the (a) anode, (b) cathode, (c) salt bridge,…
A: a) The electrode where the oxidation occurs is called as an anode. It is a negatively charged…
Q: Use the Nernst equation to show that Ecell = E°cell under standard conditions.
A: Nernst Equation: It is an equation which helps in the determination of non-standard cell potential…
Q: Draw an electrolytic cell in which Mn2+ is reduced to Mn and Snis oxidized to Sn2+. Label the anode…
A: An electrolytic cell is the electrochemical cell which converts the electrical energy into chemical…
Q: ate the cen of galvanic cell which consist of two half rea shown below
A:
Q: student constructs an electrochemical cell with a half-cell composed of a nickel anode in a 0.10 M…
A: The question is based on the concept of electrochemistry. the electrode which has higher reduction…
Q: In an experiment similar to this one, a polished strip of Cu (s) was found to weigh 16.552 g. This…
A: Given : Mass of Cu before electrolysis = 16.552 g Current passed = 0.887 A Time = 26 min = 26 X 60 =…
Q: Give three (3) examples in which scenario has electrode reactions during electrolysis. Identify…
A: i) Electrolysis of molten lead bromide. In molten state, PbBr2 exists as Pb2+ and Br- ions. On…
Q: Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced…
A:
Q: (a)electrical energy, (b), and K of the galvanic cell at
A: Galvanic cell reaction is Zn(s) + Cu2+(aq.) ---> Cu(s) + Zn2+(aq.) Cell potential(E°) at standard…
Q: A galvanic cell is constructed with a silver-silver chloride electrode, and a nickel strip immersed…
A: In the galvanic cell, at the cathode electrode, the substance will undergo reduction and the…
Q: You are asked to report the positive voltage, what type of electrochemical cell did you expect to…
A: Interpretation - Given that we are asked to report the positive voltage, what type of…
Q: A 1.00 g of metal sample took 31.05 minutes to be consumed in the anode to recharge a battery using…
A: According to Faraday's first law of electrolysis, the mass of a substance deposited on the electrode…
Q: Sketch the galvanic cell based on the following overall reactions. Calculate E°; show the direction…
A: For a cell Cell potential = E°Reduction half - E°Oxidation half Species whose reduction potential…
Q: Determine the sign of the electrodes in the cell. The left side half-cell is the and charged. The…
A: Answer: The left side of the half cell is the anode and negative charge.
Q: A Ag/Fe voltaic cell has an E° of V. If you change the concentration of the solution in each cell to…
A: The observed potential of any specified cell may or may not be identical to its standard potential.…
Q: A galvanic cell consists of Mg anode in a 1.0 M Mg(NO,), and Al cathode in a 1.0 M AI(NO,), solution…
A: The electrical energy can be calculated by using Nerst Equation
Q: For the following electrochemical cell: a)Calculate the thermodynamic potential and the free energy…
A:
Q: A galvanic cell is made of Tin anode in 1.0 M S M FeCl, solution with NaNO, as the electrolyte For…
A: The question is based on the concept of electrochemistry. the electrode which has higher reduction…
Q: (a) Write the half-reactions predicted to occur at the anode and cathode, based on the standard cell…
A:
Q: Complete the figure of the cell by labeling the anode and cathode and showing the corresponding…
A: We have to draw complete voltaiC cell.
Q: Balance each of the redox reactions for the conditions stated Label the oxidizing agent and the…
A: Answer: Unbalanced redox reaction given in problem statement is: Fe(s)+ClO4-(aq)→Fe3+(aq)+ClO3-(aq)
Q: Which statement below is not correct? O The standard hydrogen electrode has a reduction potential of…
A:
Q: standard cell potential of the reaction is 1.10 V for the cell If the Zinc ions have a…
A:
Q: A voltaic cell designed to measure (Cu**] is constructed of a standard hydrogen electrode and a…
A: A voltaic cell is an electrochemical cell in which spontaneous redox reaction occur which produces…
Q: Use the standard potential data available in the literature and design a cell with a theoretical…
A: Electrochemical cell is the device which is used to convert electrical energy into chemical energy…
Q: On the illustration of a generic cell for In/Zn electrodes immersed in their salt solutions Write…
A: Here we are required to find half cell reaction for the redox reaction which occur in the…
Q: Make your own galvanic cell. Choose a pair of electrodes (anode & cathode) from the table of…
A: In galvanic cell chemical energy is converted to electrical energy. Here we have to choose two…
Q: Part A Electrolytic cells use electricity to cause a nonspontaneous redox reaction to occur. An…
A: We have find out the answer.
Q: Calculate the potential of a lead–acid cell if all reactantsand products are in their standard…
A: Given: 1) Reactions for a lead-acid battery, The anode half-cell reaction is: Pb(s) + SO42- → PbSO4…
Q: Based upon the standard electrode potential below, which half reaction is more likely to undergo…
A: For any reaction to be a spontaneous reaction, the value of Eo should be +ve. The reactions given…
Q: A certain half-reaction has a standard reduction potential Ered=+0.90 V. An engineer proposes using…
A: It is given that E°cell ≥ 0.70 E°cat = 0.90 V
Q: 3. Does the potential difference for a half-cell reaction depend on the number of electrons that…
A:
Q: a) Electrolyte is a substance that furnishes |In the electrolytic cell, • takes place at anode and •…
A: A question based on the Faraday law concept, which is to be accomplished.
Q: A gas is observed at the cathode and the pH also went up when a solution of KBr is electrolyzed.…
A: Given: At the cathode, gas is being formed. And the pH of the solution increased when a solution of…
Q: 1. A current of A passes through an electrolytic cell for 6 mins. How many moles of electrons are…
A: If a charge Q flows through the cross-section of a conductor in time t , then we know ,…
Q: 5. How long must it take to operate a cell to obtain 3.6 g of Sn, if the electrolysis cell runs at…
A: The balanced reduction reaction for the formation of Sn(s) is: Sn2+(aq) + 2e- → Sn(s) Moles of…
Q: Salt bridge A concentration cell similar to the one shown is composed of two Al electrodes and…
A: Left compartment : anode = oxidation Right compartment : cathode = reduction Anode : Al .....>…
Q: If a current of 12A is run through an electrolysis cell for 2 hours and 10 mins, how many moles of…
A:
Q: the Relerences to access Important values Il needed lor this Salt bridge A concentration cell…
A: Since the given electrochemical cell is a concentration cell. Therefore, the E° = 0.0 V The…
Q: Which of the following statements describe the reference electrode? O electrode allows for the…
A: Reference electrode is the electrode which is used to measure the potential of other half cell…
Q: The electrolyte concentration in M at which an electrochemical cell is at its standard state.
A: The electrolyte concentration should have to be unity (1M) at its standard state
Q: na galvanic cell. a full redox reaction takes place, one half-reaction at each electrode. How is the…
A: A galvanic cell is an electrochemical cell where both oxidation and reduction process take place
Q: The standard free energy change for a reaction involving the transfer of 2 elections is -210. kJ.…
A: Given: Number of electron (n) = 2 Gibbs free energy (ΔGo) = -210 kJ
The galvanic cell test setup given in the figure is at 25 ° C. It Show the total reaction by writing the anodic and cathodic reactions of the galvanic cell. The potential to be read by the voltmeter connected to the system and the Nernst cell potential calculate.
Step by step
Solved in 2 steps

- At a constant external pressure of 43.6 bar, a mole of XX reacts via the reaction X(g)+4Y(g)→2Z(g), ΔH∘=−75.0 kJ mol−1X(g)+4Y(g)→2Z(g), ΔH∘=−75.0 kJ mol−1 Before the reaction, the volume of the gaseous mixture was 5.00 LL. After the reaction, the volume was 2.00 LL. Calculate the value of the total energy change, ΔUΔUDelta U, in kJ mol−1 kJ mol−1.A sample of potassium permaganate (KMnO4) reacts to completion with sodium oxalate (Na2C2O4) in the folowing redox reaction: 2MnO4- (aq) + 5C2O4-2 (aq) + 16H+ (aq) --> 10CO2 g) + 2Mn+2 (aq) + 8H2O (l) The volume of carbon dixoide released by the reaction at 297 K and 1.00 atm was 138 L. How many moles of potassium permanganate reacted? The Universal Gas Constant is 0.082057 L x atm/ mol x K. (answer in sig figs)One of the many remarkable enzymes in the human bodyis carbonic anhydrase, which catalyzes the interconversionof carbon dioxide and water with bicarbonate ionand protons. If it were not for this enzyme, the body couldnot rid itself rapidly enough of the CO2 accumulated bycell metabolism. The enzyme catalyzes the dehydration(release to air) of up to 107 CO2 molecules per second.Which components of this description correspond to theterms enzyme, substrate, and turnover number?
- The maximum allowable concentration of H2S(g) in air is 20mg per kilogram of air (20 ppm by mass). How many gramsof FeS would be required to react with hydrochloric acidto produce this concentration at 1.00 atm and 25 °C in anaverage room measuring 12 ft x 20 ft x 8 ft? (Under theseconditions, the average molar mass of air is 29.0 g/mol.)hello, i sent this question before but the answer you rpovided for both wasnt right and i couldnt undertstand how you got the answer. can you please show me a handwritten solution for better understanding. 1a) The decomposition of ethanol at some constant temperature (above 500°C), over a copper surface, C2H5OH(g) CH3CHO(g) + H2(g) was studied by monitoring the total pressure with time.The following data were obtained: t (s) Ptotal (torr) 0 120 45 131 139 154 233 177 376 212 380 213 What will be the total pressure at t = 462 s? 1b) What is the rate constant (k)?(Include appropriate units.)Suppose that something had gone wrong in the Big Bang, and instead of ord inary hydrogen there was an abundance of deuterium in the universe . There would be many subtle changes in equilibria, particularly the deuteron transfer equilibria of heavy atoms and bases. The Kw for D2O, heavy water, at 25 °C is 1.35 x 10-15. (a) Write the chemical equation for the autoprotolysis (more precisely, autodeuterolysis) of D2O. (b) Evaluate pKw for D2O at 25 ° C. (c) Calculate the molar concentrations of D3O+ and OD- in neutral heavy water at 25 °C . (d) Evaluate the pD and pOD of neutral heavy water at 25 °C. (e) Formulate the relation between pD, pOD, andpKw(D2O).
- PV = nRT. The pressure is 0.9912atm. V = 50.5 mL. T = 21.5°C and n = 0.00200mol. Calculate R from this data in Latm/molK.The atomic hydrogen exists in space at an estimated concentration of one particle per cubic meter. If the collision diameter is 2.5 ×10^(–10) meter and the temperature is 2.7 Kelvin, how many kilometers away will the next potential collision be? Express the answer in three significant figures.The global emissions of methane have been estimated to be near 9,390 million metric tons of CO2 equivalent, assuming there is a GWP of 25. Calculate the residence of methane in the atmosphere. If the concentration of methane in the atmosphere is 1.55 ppmv. Can an expert, please show me how to solve the question above?
- Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07For the reduction 2FeCl3 + SnCl2 =====➔ 2 FeCl2 + SnCl4 in aqueous solution the following data were obtained at 25oC t(min) 1 3 7 11 40 Y 0.01434 0.02664 0.03612 0.04102 0.05058 Where y is the amount of FeCl3 reacted in moles per liter. The initial concentrations of SnCl3 and FeCl3 were respectively, 0.03125, 0.0625 moles/L. a.)Show that the reaction is third order (derive the rate law), and b.) calculate the average specific rate constant.I AM NOT A NATIVE ENGLISH SPEAKER, PLS WRITE IT CLEAR. The following is a formulation of the decomposition of penta-nitrogen dioxide in 456K N2O5(g) → 2NO2(g) +1\2O2(g) ? = 6.35 × 10−3?−1 A 0.18 liter hard vessel located in the 456K was inserted N2O5(G) At a pressure of 1.22 atmospheres. the reaction above happend. 1. How long from the start of the experiment will the pressure in the system rise to 1.54 atmospheres? 2. Calculate the partial pressure of each component in the mixture 100 seconds from the start of the experiment. 3. It was found that at a temperature of K 400 the value of the rate constant for a decomposition reaction of a nitrogen Fanta-oxygen equals 5.42*10-3 sec-1. Calculate the operating energy for the reaction. in all sections show calculations.



