the give and sodium chloride, and then write the net ionic equation. Show states for all reactants and products (s, C, g, aq). The "alum" used in cooking is potassium aluminum sulfate hydrate, KAI(SO.)2 x H20. To find the value of x, you can heat a sample of the compound to drive off all of the water and leave only KAI(SO.)2. Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g of water. What is the value of x? Hydrogen cyanide is a highly poisonous, volatile liquid. One way in which it can be prepared is by the reaction:
the give and sodium chloride, and then write the net ionic equation. Show states for all reactants and products (s, C, g, aq). The "alum" used in cooking is potassium aluminum sulfate hydrate, KAI(SO.)2 x H20. To find the value of x, you can heat a sample of the compound to drive off all of the water and leave only KAI(SO.)2. Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g of water. What is the value of x? Hydrogen cyanide is a highly poisonous, volatile liquid. One way in which it can be prepared is by the reaction:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 52QAP: Write a balanced equation for the reaction between (a) dihydrogen sulfide and sulfur dioxide gases...
Related questions
Question
5 and 7 please
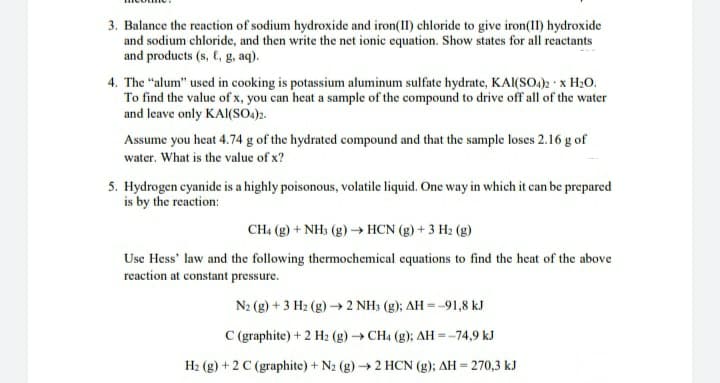
Transcribed Image Text:3. Balance the reaction of sodium hydroxide and iron(II) chloride to give iron(1I) hydroxide
and sodium chloride, and then write the net ionic equation. Show states for all reactants
and products (s, C, g, aq).
4. The "alum" used in cooking is potassium aluminum sulfate hydrate, KAl(SO.): x H20.
To find the value of x, you can heat a sample of the compound to drive off all of the water
and leave only KAI(SO.)2.
Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g of
water. What is the value of x?
5. Hydrogen cyanide is a highly poisonous, volatile liquid. One way in which it can be prepared
is by the reaction:
CH4 (g) + NH3 (g)→ HCN (g) + 3 H2 (g)
Use Hess' law and the following thermochemical equations to find the heat of the above
reaction at constant pressure.
N2 (g) + 3 H2 (g) → 2 NH3 (g); AH = -91,8 kJ
C (graphite) + 2 H2 (g)→ CH4 (g); AH=-74,9 kJ
H2 (g) + 2 C (graphite) + N2 (g)→2 HCN (g); AH = 270,3 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning