The half life for the radioactive decay of potassium-40 to argon-40 is 1.26 x 10 years. Suppose nuclear chemical analysis shows that there is 0.727 mmol of argon-40 for every 1.000 mmol of potassium-40 in a certain sample of rock. Calculate the age of the rock. Round your answer to 2 significant digits. O years
The half life for the radioactive decay of potassium-40 to argon-40 is 1.26 x 10 years. Suppose nuclear chemical analysis shows that there is 0.727 mmol of argon-40 for every 1.000 mmol of potassium-40 in a certain sample of rock. Calculate the age of the rock. Round your answer to 2 significant digits. O years
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 19E: The rate constant for the radioactive decay of 14C is 1.21104 year-1. The products of the decay are...
Related questions
Question
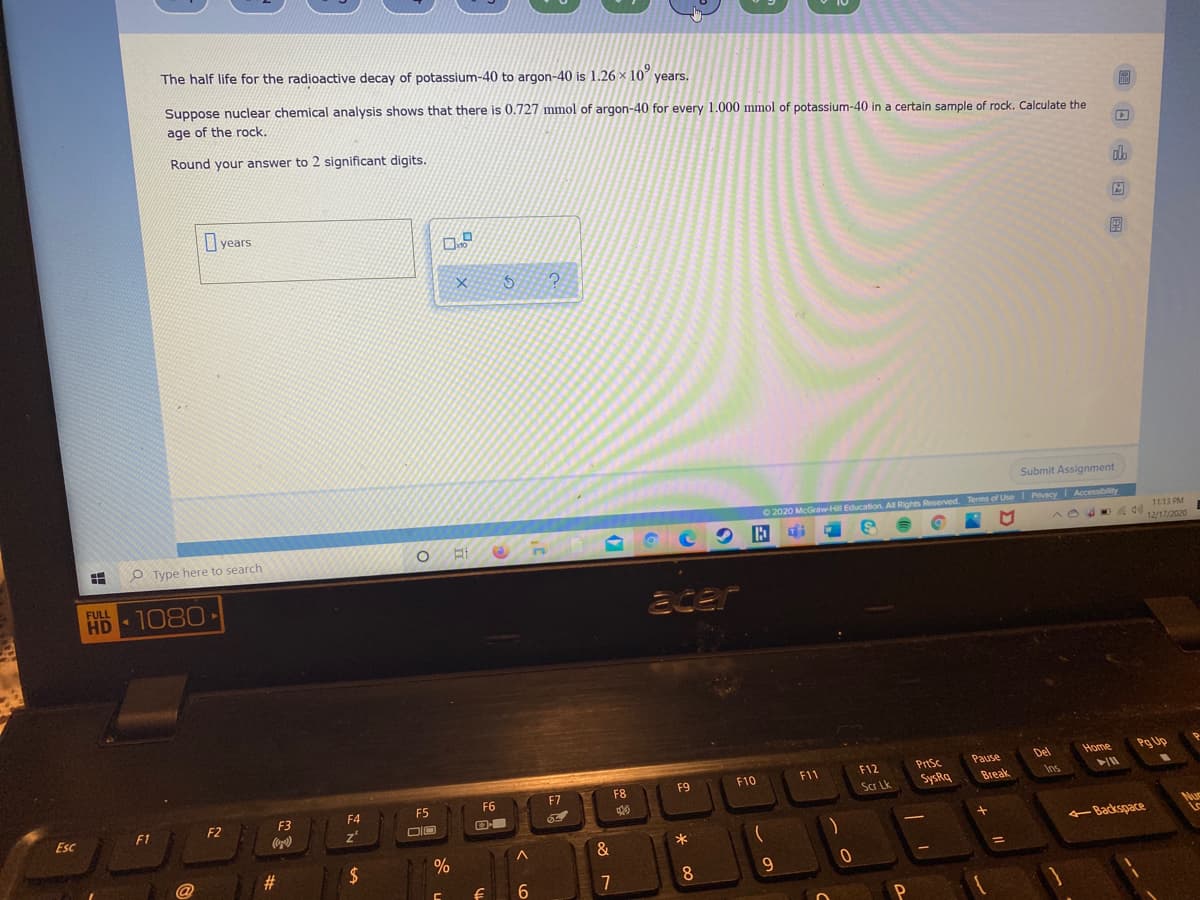
Transcribed Image Text:The half life for the radioactive decay of potassium-40 to argon-40 is 1.26 × 10 years.
Suppose nuclear chemical analysis shows that there is 0.727 mmol of argon-40 for every 1.000 mmol of potassium-40 in a certain sample of rock. Calculate the
age of the rock.
Round your answer to 2 significant digits.
db
years
Submit Assignment
O 2020 McGraw-Hi Education. All Rights Reserved. Terms of Uhe I Pivacy I Accessibility
1113 PM
0 49 12/17/2020
O Type here to search
FULL
HD 1080
Ecer
Del
Pg Up
Pause
Home
F11
F12
PrtSc
F6
F7
F8
F9
F10
Sa Lk
SysRq
Break
Ins
F2
F3
F4
F5
Esc
F1
63
z'
Nu
&
Backspace
@
#
7
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning