The half-life of 14C is 5730 years, and the current decay rate in living organisms is 14.3 disintegrations per minute per gram of carbon (d.p.m./g C). What is the age (in years BP) of an artifact found to have a decay rate of 4.75 d.p.m/g C in 2013?
The half-life of 14C is 5730 years, and the current decay rate in living organisms is 14.3 disintegrations per minute per gram of carbon (d.p.m./g C). What is the age (in years BP) of an artifact found to have a decay rate of 4.75 d.p.m/g C in 2013?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter18: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 28E
Related questions
Question
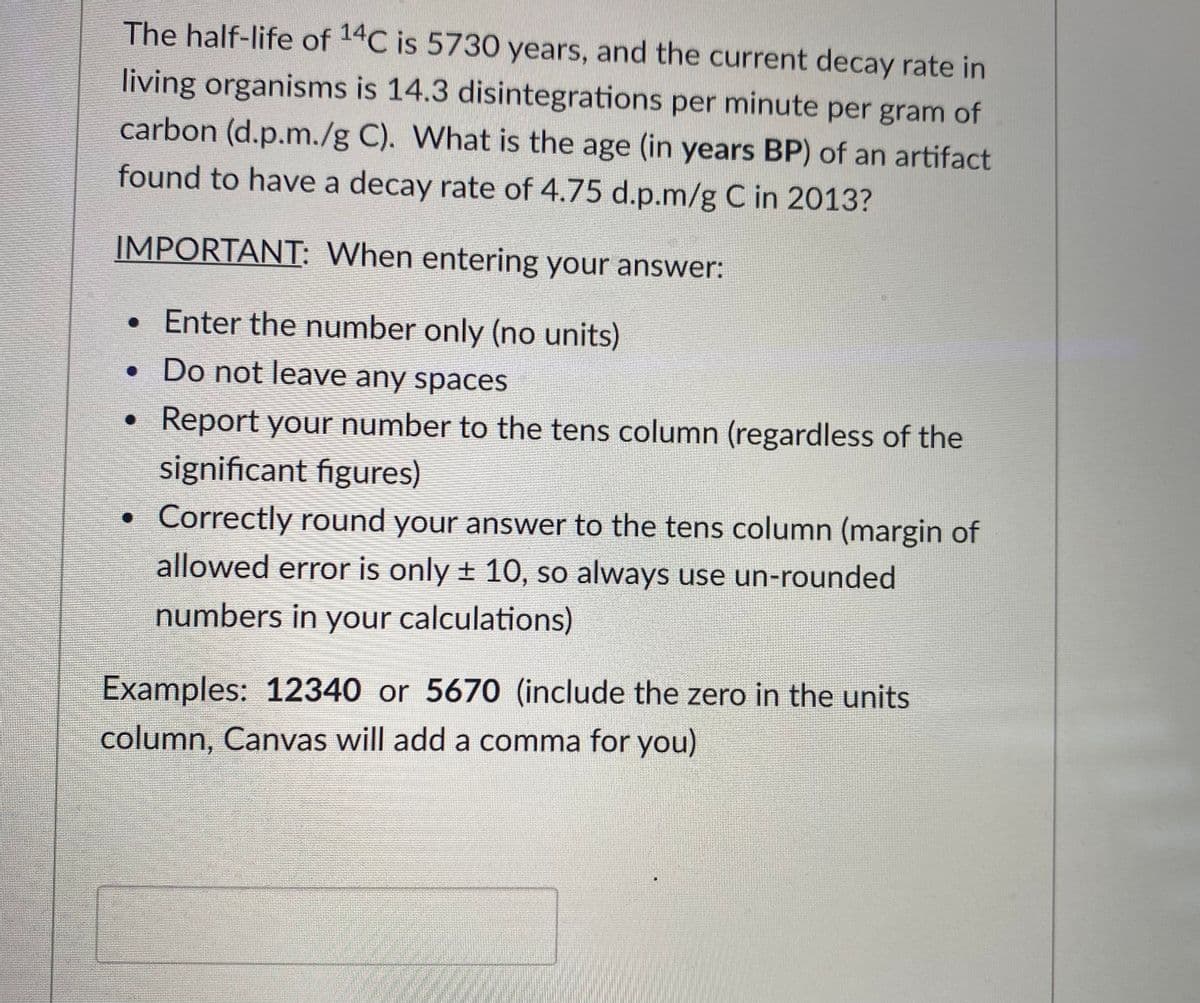
Transcribed Image Text:The half-life of 14C is 5730 years, and the current decay rate in
living organisms is 14.3 disintegrations per minute per gram of
carbon (d.p.m./g C). What is the age (in years BP) of an artifact
found to have a decay rate of 4.75 d.p.m/g C in 2013?
IMPORTANT: When entering your answer:
• Enter the number only (no units)
• Do not leave any spaces
• Report your number to the tens column (regardless of the
significant figures)
Correctly round your answer to the tens column (margin of
allowed error is only ± 10, so always use un-rounded
numbers in your calculations)
Examples: 12340 or 5670 (include the zero in the units
column, Canvas will add a comma for you)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning