The heating element of a coffeemaker operates at 120 V and carries a current of 3.70 A. Assuming the water absorbs all of the energy converted by the resistor, calculate how long it takes to heat 0.692 kg of water from room temperature (23.0°C) to the boiling point. Step 1 The energy required to raise the temperature of an amount of water of mass m, from T, = 23.0°C to the boiling point, T = 100°C, is Q = m„C„(AT), where the specific heat of water c, = 4186 J/kg · °C. We have Q = mCw(AT) = mC(T - T) .692 0.692 kg)(4186 J/kg · °C)(77v 7기 0C 3D 2.23 V 2.23 x 105 J. Step 2 The rate P at which the heating element converts electrical potential energy into the internal energy of the water is P = (AV)I = 120 120 V 3.7 3.7 A) = 444 V 444 J/s.
The heating element of a coffeemaker operates at 120 V and carries a current of 3.70 A. Assuming the water absorbs all of the energy converted by the resistor, calculate how long it takes to heat 0.692 kg of water from room temperature (23.0°C) to the boiling point. Step 1 The energy required to raise the temperature of an amount of water of mass m, from T, = 23.0°C to the boiling point, T = 100°C, is Q = m„C„(AT), where the specific heat of water c, = 4186 J/kg · °C. We have Q = mCw(AT) = mC(T - T) .692 0.692 kg)(4186 J/kg · °C)(77v 7기 0C 3D 2.23 V 2.23 x 105 J. Step 2 The rate P at which the heating element converts electrical potential energy into the internal energy of the water is P = (AV)I = 120 120 V 3.7 3.7 A) = 444 V 444 J/s.
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter27: Direct-current Circuits
Section27.4: Rc Circuits
Problem 27.5QQ: Consider the circuit in Figure 27.17 and assume the battery has no internal resistance. (i) Just...
Related questions
Question
Transcribed Image Text:Tutorial Exercise
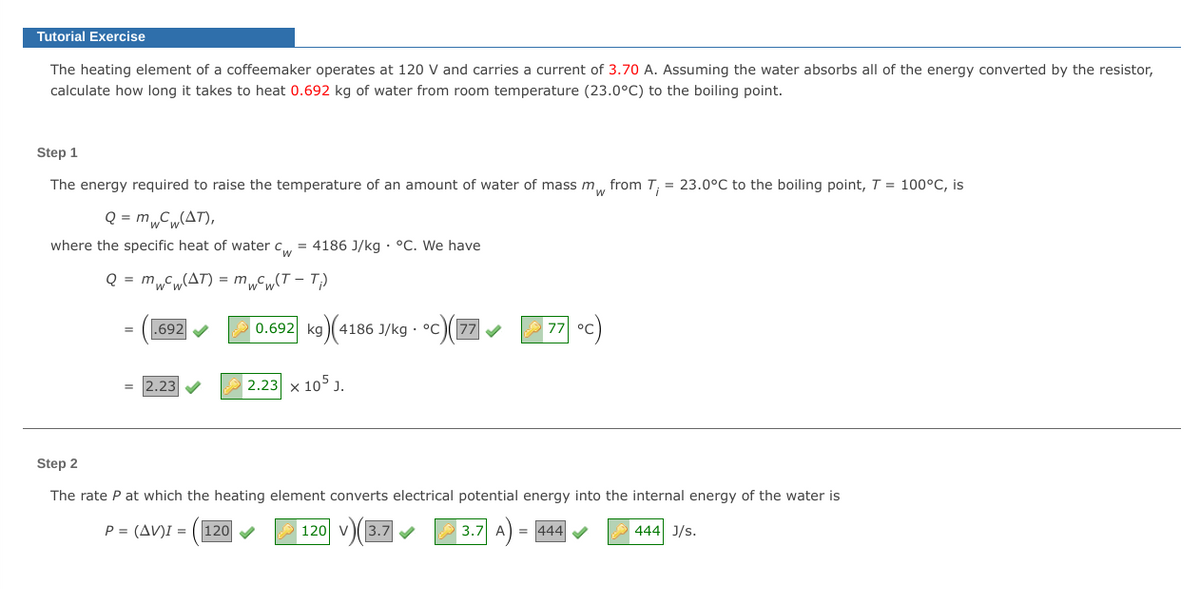
The heating element of a coffeemaker operates at 120 V and carries a current of 3.70 A. Assuming the water absorbs all of the energy converted by the resistor,
calculate how long it takes to heat 0.692 kg of water from room temperature (23.0°C) to the boiling point.
Step 1
The energy required to raise the temperature of an amount of water of mass m, from T, = 23.0°C to the boiling point, T = 100°C, is
Q = m„C„(AT),
where the specific heat of water c,, = 4186 J/kg · °C. We have
Q = mCw(AT) = m„Cw(T – T)
692 V
0.692 kg)(41
4186 J/kg · °c)7
77
77
°C
= 2.23
2.23 x 105 J.
Step 2
The rate P at which the heating element converts electrical potential energy into the internal energy of the water is
P = (AV)I = ( 120
120 v
3.7
3.7 A
= 444 V
444 J/s.
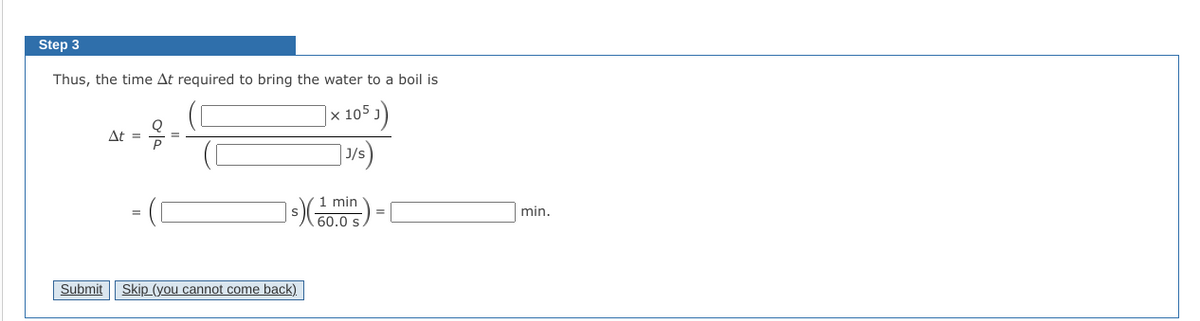
Transcribed Image Text:Step 3
Thus, the time At required to bring the water to a boil is
|x 105 )
At =
J/s
1 min
min.
60.0 s.
Submit
Skip (you cannot come back)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning