The hydrogen ion concentrationH* of blood is approximately 4.18 ×10-8 moles per liter. Find the pH value of human blood. Is human blood acidic or basic? The pH value of human blood is approximately. (Simplify your answer. Round to the nearest tenth as needed.)
The hydrogen ion concentrationH* of blood is approximately 4.18 ×10-8 moles per liter. Find the pH value of human blood. Is human blood acidic or basic? The pH value of human blood is approximately. (Simplify your answer. Round to the nearest tenth as needed.)
Chapter9: Math Models And Geometry
Section9.6: Solve Geometry Applications: Volume And Surface Area
Problem 9.111TI: TRYIT:: 9,111 How many cubic inches of candy will fit in a cone-shaped piñata that is 18 inches long...
Related questions
Question
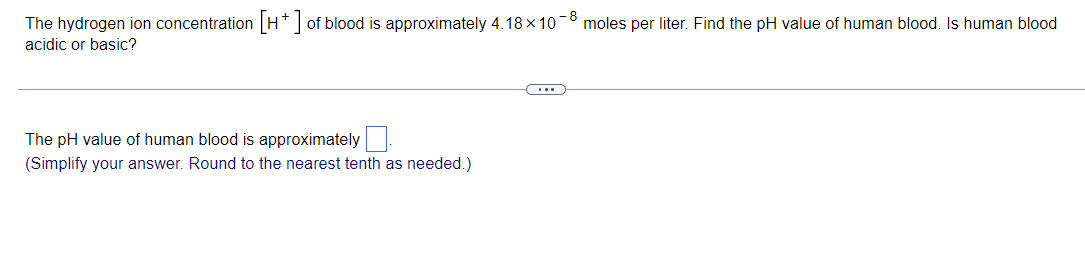
Transcribed Image Text:The hydrogen ion concentrationH* of blood is approximately 4.18 ×10-8 moles per liter. Find the pH value of human blood. Is human blood
acidic or basic?
The pH value of human blood is approximately
(Simplify your answer. Round to the nearest tenth as needed.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,



Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,


Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Intermediate Algebra
Algebra
ISBN:
9781285195728
Author:
Jerome E. Kaufmann, Karen L. Schwitters
Publisher:
Cengage Learning