The ideal gas law PV = nRT gives a relationship between the pressure P in pascals (you can read about the pressure unit pascals here if you'd like 2 ), the volume V in cubic meters, n is the number of moles of a substance present and R is Avogadro's number (you can read about moles and Avogadro's number here if you'd like 2 ) and T is the temperature in Kelvins (here's some information about the Kelvin scale) e. It's worth mentioning that the ideal gas law assumes we can ignore things like the volume of individual molecules and the attraction between particles. So if we say something like "Pretend we have an ideal gas in a box," we are quite literally pretending, because there is no ideal gas! But this equation is a statement about the relationship of units to each other, and thus is useful for comparing quantities in an approximate way. In practice, chemists might measure the deviation from the ideal gas law in order to deduce information about other properties. The tools we developed in this unit for comparing related rates can help us analyze situations involving the ideal gas equation. • Use implicit differentiation to differentiate the ideal gas law equation with respect to time. (Hint: Two quantities are always constant, which ones?)
The ideal gas law PV = nRT gives a relationship between the pressure P in pascals (you can read about the pressure unit pascals here if you'd like 2 ), the volume V in cubic meters, n is the number of moles of a substance present and R is Avogadro's number (you can read about moles and Avogadro's number here if you'd like 2 ) and T is the temperature in Kelvins (here's some information about the Kelvin scale) e. It's worth mentioning that the ideal gas law assumes we can ignore things like the volume of individual molecules and the attraction between particles. So if we say something like "Pretend we have an ideal gas in a box," we are quite literally pretending, because there is no ideal gas! But this equation is a statement about the relationship of units to each other, and thus is useful for comparing quantities in an approximate way. In practice, chemists might measure the deviation from the ideal gas law in order to deduce information about other properties. The tools we developed in this unit for comparing related rates can help us analyze situations involving the ideal gas equation. • Use implicit differentiation to differentiate the ideal gas law equation with respect to time. (Hint: Two quantities are always constant, which ones?)
Functions and Change: A Modeling Approach to College Algebra (MindTap Course List)
6th Edition
ISBN:9781337111348
Author:Bruce Crauder, Benny Evans, Alan Noell
Publisher:Bruce Crauder, Benny Evans, Alan Noell
Chapter2: Graphical And Tabular Analysis
Section2.4: Solving Nonlinear Equations
Problem 2TU
Related questions
Question
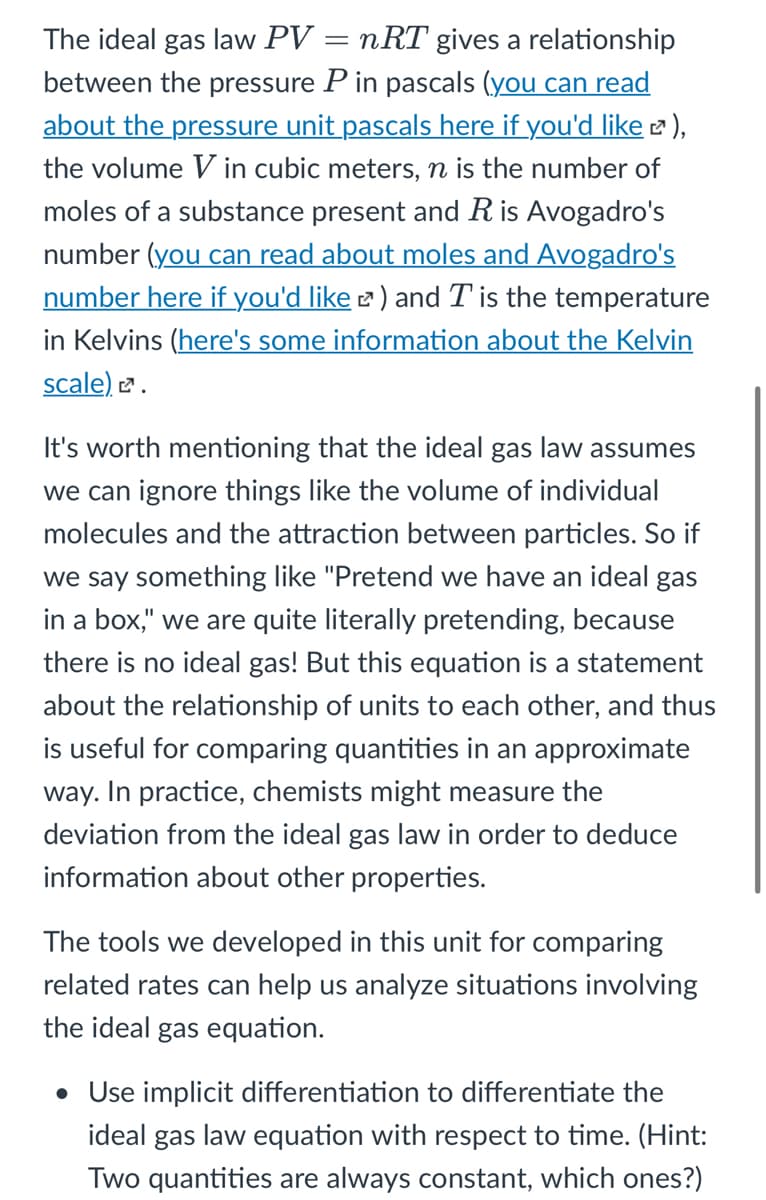
Transcribed Image Text:The ideal gas law PV = nRT gives a relationship
between the pressure P in pascals (you can read
about the pressure unit pascals here if you'd like ),
the volume V in cubic meters, n is the number of
moles of a substance present and Ris Avogadro's
number (you can read about moles and Avogadro's
number here if you'd like 2 ) and T is the temperature
in Kelvins (here's some information about the Kelvin
scale) 2.
It's worth mentioning that the ideal gas law assumes
we can ignore things like the volume of individual
molecules and the attraction between particles. So if
we say something like "Pretend we have an ideal gas
in a box," we are quite literally pretending, because
there is no ideal gas! But this equation is a statement
about the relationship of units to each other, and thus
is useful for comparing quantities in an approximate
way. In practice, chemists might measure the
deviation from the ideal gas law in order to deduce
information about other properties.
The tools we developed in this unit for comparing
related rates can help us analyze situations involving
the ideal gas equation.
• Use implicit differentiation to differentiate the
ideal
gas law equation with respect to time. (Hint:
Two quantities are always constant, which ones?)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

