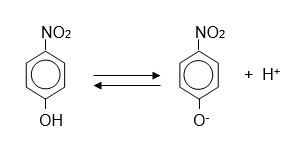
The ionization of p-nitrophenol is shown below (pKa= 7.0) a) At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol? b) If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms? c) A solution of p-nitrophenol at pH 8.3 was found to have an A400 of 0.550. What is the total concentration (in um) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 19,500M-1cm-1 and the pKa is 7.
The ionization of p-nitrophenol is shown below (pKa= 7.0) a) At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol? b) If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms? c) A solution of p-nitrophenol at pH 8.3 was found to have an A400 of 0.550. What is the total concentration (in um) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 19,500M-1cm-1 and the pKa is 7.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 61QAP: If a reaction occurs readily but has an endothermic heat of reaction, what must be the driving force...
Related questions
Question
The ionization of p-nitrophenol is shown below (pKa= 7.0)
a) At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol?
b) If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms?
c) A solution of p-nitrophenol at pH 8.3 was found to have an A400 of 0.550. What is the total concentration (in um) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 19,500M-1cm-1 and the pKa is 7.
Transcribed Image Text:NO2
ОН
NO₂
O-
+ H+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning