Q: O 0.0001
A:
Q: If you compare orbitals with different principal quantum numbers (n) but the same angular momentum (...
A:
Q: It takes a minute and a half for the concentration of oxygen to decrease from 4.2 x10 2 M to 2.4 x10...
A:
Q: 03) Below are presented two 1H NMR spectra. Among the compounds pentan-3-ol, pentan2-ol, pent-3-amin...
A: HNMr tells us about the spiltting pattern of hydrogens in the compound.
Q: Which of the following substances contain a rigid arrangement of their constituent molecules? Sugar ...
A: arrangement of molecule in compound decide it's rigidity if molecule are close to each other then co...
Q: Based on the Lewis structure of the nitrate ion (NO3 ), how many lone pair(s) is(are) on the central...
A:
Q: Which of the following (NBR3, BF3, OF2, IF3) is(are) exempted from the octet rule? Check all that ap...
A: Generally octet rule is violated or exempted when a central atom having either fewer or more than th...
Q: Calculate the percent by mass of potassium nitrate in a solution made from 67.0 g KN0, and 447 mL of...
A: Given: Mass of KNO3 = 67 g The volume of water = 447 mL The density of water = 0.997 g/mL Standard...
Q: Give the number of lone pairs around the central atom and the geometry of NO3. O 0; trigonal planar ...
A:
Q: Synthesize the compund (Amlodipine) via Hantzsch dihydropyridine synthesis
A: The answer with mechanism is as follows:
Q: Vhat is the minimum amount of heat required to completely melt 15.0 grams of ice at its nelting poin...
A:
Q: H2O H, Heat
A:
Q: an aqueous solution has a pOH = 6.8. The pH of this solution is ____
A: • The values provided in the question are:- i) pOH of an aqueous solution = 6.8 • We need to...
Q: 2) What is true about the relationship of Kp and Kc for the reaction: 2 CH4(g) + 3 O2(g)=2 CO(g) + 4...
A:
Q: For the reaction below which of the statements is correct? A+ 2B → 3C O The reaction is second-order...
A: Rate law is determined by slow step of the mechanism of the reaction.
Q: hat is the reaction equation for copper (II) and thiosulfate ions and the completed reaction of 2Fe(...
A: Given : We have to complete the given reaction.
Q: how many moles of carbon atoms are in 2.87×10^22 molecules of compound C5H6O2
A: Given Molecules of C5H6O2 = 2.87 × 1022
Q: 1. A tank is filled with 1,040 liters of oil. If the specific weight of oil is 8760 N/m³, determine ...
A:
Q: H2 (1-equiv) Pd-C [1] CH3LI но но [2] H2O [1] (CH3)2CuLi OH [2] H20 [1] CH3CH2MGB OH [2] H2O H2 (exc...
A: We have to tell the products formed on the given reactions.
Q: e enthalpy change
A:
Q: Which compound has the greatest ionic character? a. CsF b. LiF c. NaF d. RbF
A:
Q: Refer to the phase diagram below when answering the questions on this worksheet. Begin by adding S, ...
A: The phase diagram given is,
Q: how many moles of alum will be produced if a student reacts 0.25 moles of aluminum with 0.35 moles o...
A: Given: Moles of Al = 0.25 mol. Moles of KOH = 0.35 mol. And moles of H2SO4 = 0.76 mol.
Q: Total number of H-bonds formed by water molecules in 'liquid' state are a 4 b 3 c 2 d ...
A: To identify: The number of hydrogen bonds formed by the water molecules in the liquid state.
Q: 8. Calculate the mole fraction of water in the solution described in Q5. The density of pure water a...
A: Solving 8 9 and 10
Q: Which of the following molecules can only have dispersion forces as their sole intermolecular force ...
A: Given : We have to tell which of the following molecule will have dispersion forces of attraction.
Q: weights önly honpolar and non-lonic molecules consisting of only hydrogen and carbon, which of the f...
A: The correct option is:
Q: Which of the following molecules is polar? O BeCl2 O CH2F2 O CH4 O AIF3
A: Given : We have to write which of the following is polar.
Q: chemical équation 0/5 A major component of gasoline is octane (C3H18). When octane is burned in air,...
A:
Q: 1) What is the equilibrium equation for the FeS(s) + 2 H30+ (aq) = Fe2+(aq) + H2S (aq) + 2 H20 (1)
A: Since you have posted multiple questions as per guidelines we can solve only one per session . If yo...
Q: If the following molecules satisfy the octet rule, write "stable." If they do not, write "unstable."...
A: Every molecule wants to be stable like inert gas. Inert gases have eight electrons in its valence Sh...
Q: Determine the volume of solution that contains 15.0g of AgNO3 if the solution is 0.375M a. 247mL b....
A:
Q: for å first order decomposition reaction, → products, given the time needed to decrease the initial ...
A:
Q: Which of the following compound has the central atom with +1 formal charge? O PCI6 O XEF3* O IF5 O A...
A:
Q: How many moles of NaOH are present in 25.0 mL of a 0.1000 M NaOH solution? O 2.50 mol O 0.100 mol O ...
A:
Q: If 0.5635g of xenon reacted with 0.4893g of fluorine, calculate the empirical formula of the compoun...
A: Given, 0.5635 g of xenon reacted with 0.4893 g of fluorine.
Q: On acidity and basicity of aqueous solutions, the following relationships are valid, except [H+][-OH...
A: Given : We have to tell the incorrect statement.
Q: A piece of aluminum with a mass of 23.5 grams at a temperature of 0.0°C is dropped into an insulated...
A: Mass of Aluminium = 23.5 Final temperature = 87.2°C
Q: H3C H3C 2 H3C \H3C CuLi Li H3C CI OH H;C H;CO H3C D Br MgBr H3CO H3CO H3CO 2.
A: The given reaction involves multi-step. All steps are significant. This is a multi-step transformati...
Q: Briefly discuss the difference between a homogenous mixture and a heterogeneous mixture.
A: When different substances mix to form a uniform mixture in same phase , then it is called homogeneou...
Q: Which of these compounds is most soluble in water at pH 7.0? (Note that the non-ionized forms are sh...
A:
Q: at mass of oxyg
A:
Q: The pH for 0.0850 M solution of C6H:CH2COOH is 2.68. Determine the value of Ka for CsHsCH2COOH. PREV...
A:
Q: Which of the following mixtures would result in buffered solutions when 1.0 L of each of the two sol...
A:
Q: Given the following peptide RISAGDLEVK, Determine the formal charge of peptide a. pH=0.5 b. pH=...
A:
Q: Enter your answer in the provided box. A sample of 8.61 g of naphthalene (C1,Hg) is dissolved in 75....
A:
Q: It takes 42.0 min for the concentration of a reactant in a first-order reaction to drop from 0.45 M ...
A: Given, Time, t = 42 min Initial Concentration [A]0 = 0.45 M Final Concentration, [A] = 0.32 M
Q: 1) BH3-THF 2) H2О2, NaOН Na2Cr207 H2SO4, H20 но OH (2 equiv)
A: Since you have posted questions with multiple sub-parts, we are entitled to answer the first 3 only....
Q: Which of these compounds is most soluble in water at pH 7.0? (Note that the non-ionized forms are sh...
A:
Q: the rate at
A:

![For the reaction in question 3, what will be the free energy under the
following conditions?
[Ni2*] = 0.0010M
[NH3] = 0.0050 M
[Ni(NH3),2*] = 0.010 M
Give me AG in J/mol?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ffd47ee59-8c02-4471-82b7-8a3c00daed6e%2F459a8113-2b3b-459b-8598-01c5ed4ea414%2F4m5agt6_processed.png&w=3840&q=75)
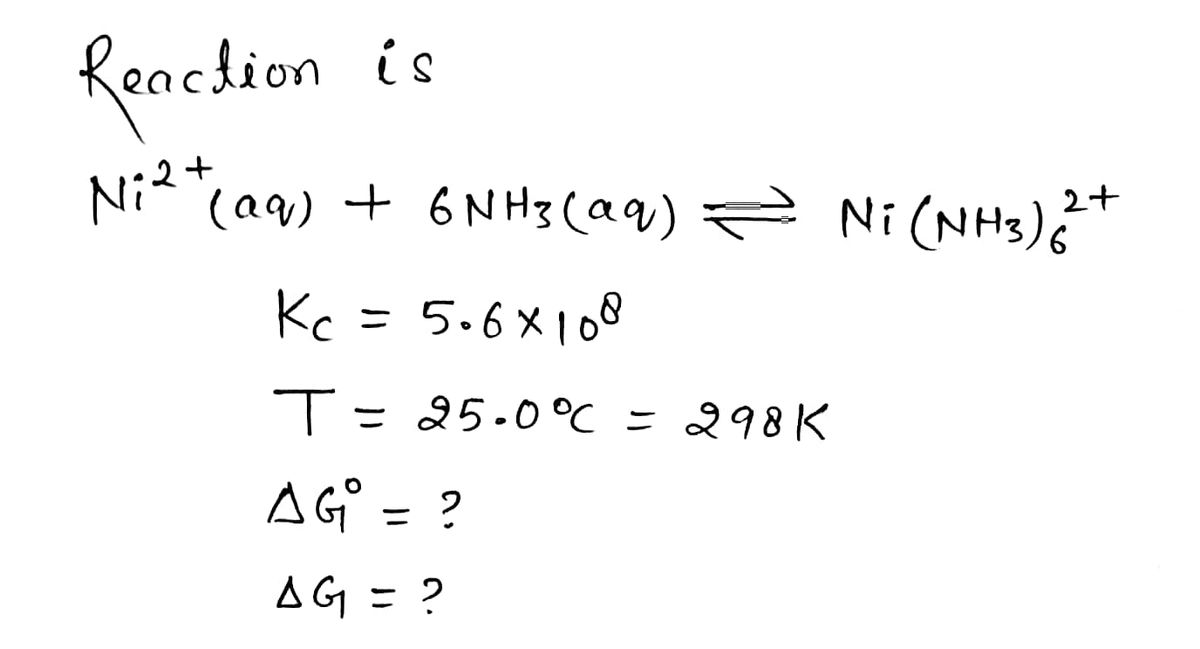
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
