The law of atmospheres states that the number density of molecules in the atmosphere depends on height y above sea level according to where n, is the number density at sea level (where y = 0). The average height of a molecule in the Earth's atmosphere is given by | yn, (1) dy ye D/,T dy avg |n,G) dy eD/A,T dy (a) Prove that this average height is equal to kT/m,g. (b) Evaluate the average height, assuming the temperature is 10.0°C and the molecular mass is 28.9 u, both uniform throughout the atmosphere.
The law of atmospheres states that the number density of molecules in the atmosphere depends on height y above sea level according to where n, is the number density at sea level (where y = 0). The average height of a molecule in the Earth's atmosphere is given by | yn, (1) dy ye D/,T dy avg |n,G) dy eD/A,T dy (a) Prove that this average height is equal to kT/m,g. (b) Evaluate the average height, assuming the temperature is 10.0°C and the molecular mass is 28.9 u, both uniform throughout the atmosphere.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 52P: The partial pressure of carbon dioxide in the lungs is about 470 Pa when the total pressure in the...
Related questions
Question
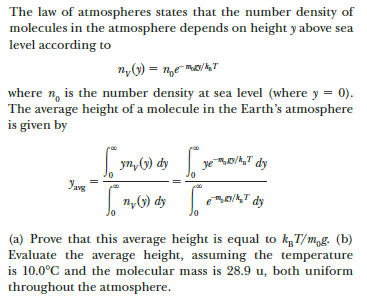
Transcribed Image Text:The law of atmospheres states that the number density of
molecules in the atmosphere depends on height y above sea
level according to
where n, is the number density at sea level (where y = 0).
The average height of a molecule in the Earth's atmosphere
is given by
| yn, (1) dy
ye D/,T dy
avg
|n,G) dy
eD/A,T dy
(a) Prove that this average height is equal to kT/m,g. (b)
Evaluate the average height, assuming the temperature
is 10.0°C and the molecular mass is 28.9 u, both uniform
throughout the atmosphere.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 9 images

Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College