The mendelic acid "chemical face peel" is a relatively new innovation for the rejuvenation of problem skin. In addition to removing dead skin cells, its antibacterial properties help acne-prone skin. The technique has also been touted as a way to improve hyperpigmentation due to post acne scarring. Although the compound was first derived from bitter almonds, mendelic acid is generally now synthesized in the laboratory. To formulate one of these "face peel" emulsions, you are asked to prepare as much pH = 3.543 buffer solution as you can from 100.00 mL of 0.5000 M mendelic acid (HMend). Since the use of mendelic acid is relatively new, the conjugate base mendolate (Mend') cannot be purchased. Fortunately, you have plenty of 0.4018 M NaOH. Ka = 3.894× 10-4 for mendelic acid. How would you prepare the buffer solution? The following questions will guide you to find the final answer. A. What is the moles ratio between the equilibrium molarity of mendelic acid (MA) and mendolate?
The mendelic acid "chemical face peel" is a relatively new innovation for the rejuvenation of problem skin. In addition to removing dead skin cells, its antibacterial properties help acne-prone skin. The technique has also been touted as a way to improve hyperpigmentation due to post acne scarring. Although the compound was first derived from bitter almonds, mendelic acid is generally now synthesized in the laboratory. To formulate one of these "face peel" emulsions, you are asked to prepare as much pH = 3.543 buffer solution as you can from 100.00 mL of 0.5000 M mendelic acid (HMend). Since the use of mendelic acid is relatively new, the conjugate base mendolate (Mend') cannot be purchased. Fortunately, you have plenty of 0.4018 M NaOH. Ka = 3.894× 10-4 for mendelic acid. How would you prepare the buffer solution? The following questions will guide you to find the final answer. A. What is the moles ratio between the equilibrium molarity of mendelic acid (MA) and mendolate?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.64P
Related questions
Concept explainers
Question
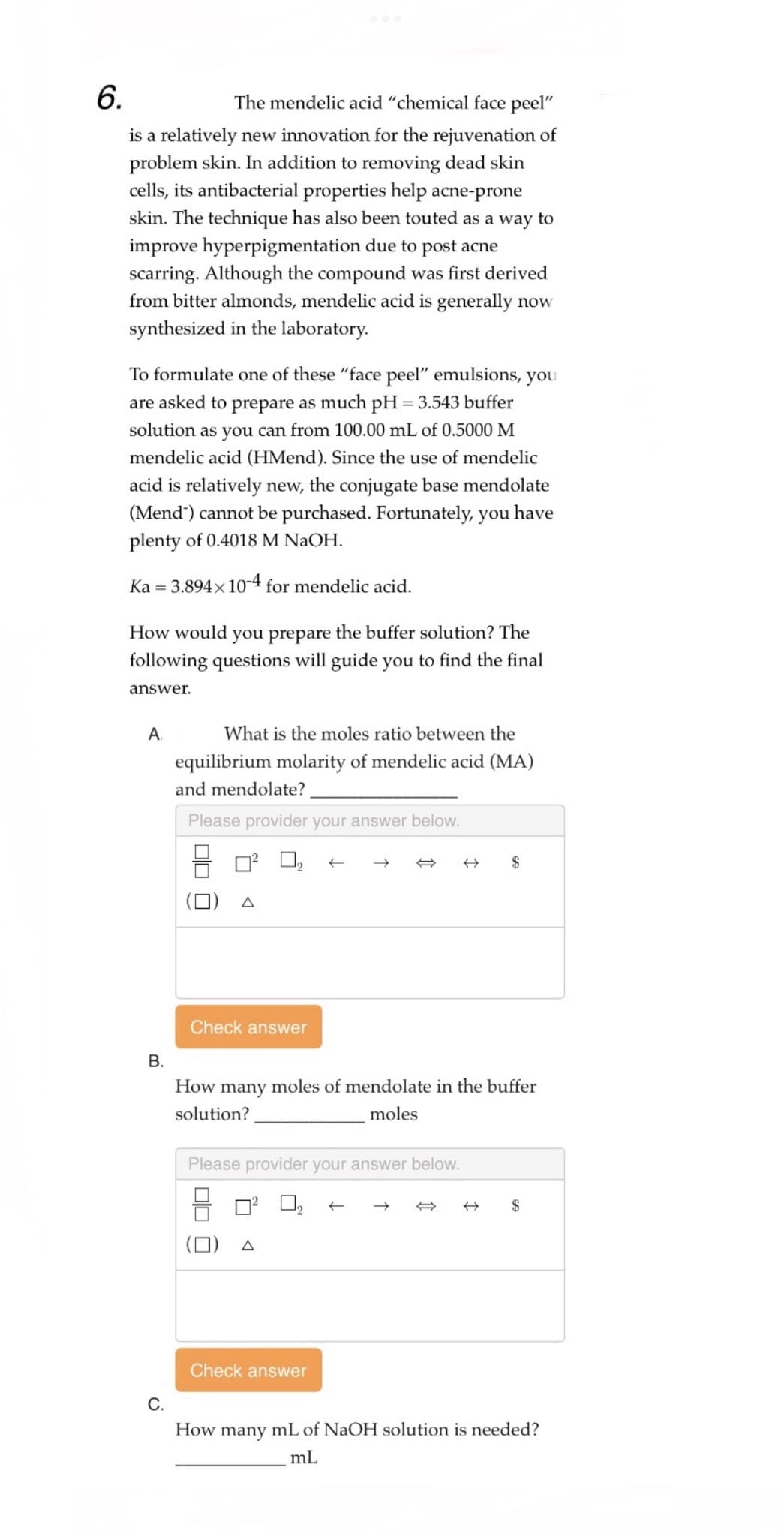
Transcribed Image Text:6.
The mendelic acid "chemical face peel"
is a relatively new innovation for the rejuvenation of
problem skin. In addition to removing dead skin
cells, its antibacterial properties help acne-prone
skin. The technique has also been touted as a way to
improve hyperpigmentation due to post acne
scarring. Although the compound was first derived
from bitter almonds, mendelic acid is generally now
synthesized in the laboratory.
To formulate one of these "face peel" emulsions, you
are asked to prepare as much pH = 3.543 buffer
solution as you can from 100.00 mL of 0.5000 M
mendelic acid (HMend). Since the use of mendelic
acid is relatively new, the conjugate base mendolate
(Mend) cannot be purchased. Fortunately, you have
plenty of 0.4018 M NaOH.
Ka = 3.894x 10-4 for mendelic acid.
How would you prepare the buffer solution? The
following questions will guide you to find the final
answer.
A
B.
C.
What is the moles ratio between the
equilibrium molarity of mendelic acid (MA)
and mendolate?
Please provider your answer below.
00
A
2
Check answer
A
How many moles of mendolate in the buffer
solution?
moles
Please provider your answer below.
→> ←
Check answer
←
$
$
How many mL of NaOH solution is needed?
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

