The molar heat capacity of silver is 25.35 J mol-1 °C-!. How much energy would it take to raise the temperature of 11.4 g of silver by 11.8 °C ?
The molar heat capacity of silver is 25.35 J mol-1 °C-!. How much energy would it take to raise the temperature of 11.4 g of silver by 11.8 °C ?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.104PAE: 9.104 An engineer is using sodium metal as a cooling agent in a design because it has useful thermal...
Related questions
Question
PLEASE HELP AND DO BOTH OF THEM AS THEY ARE PART OF 1 QUESTION
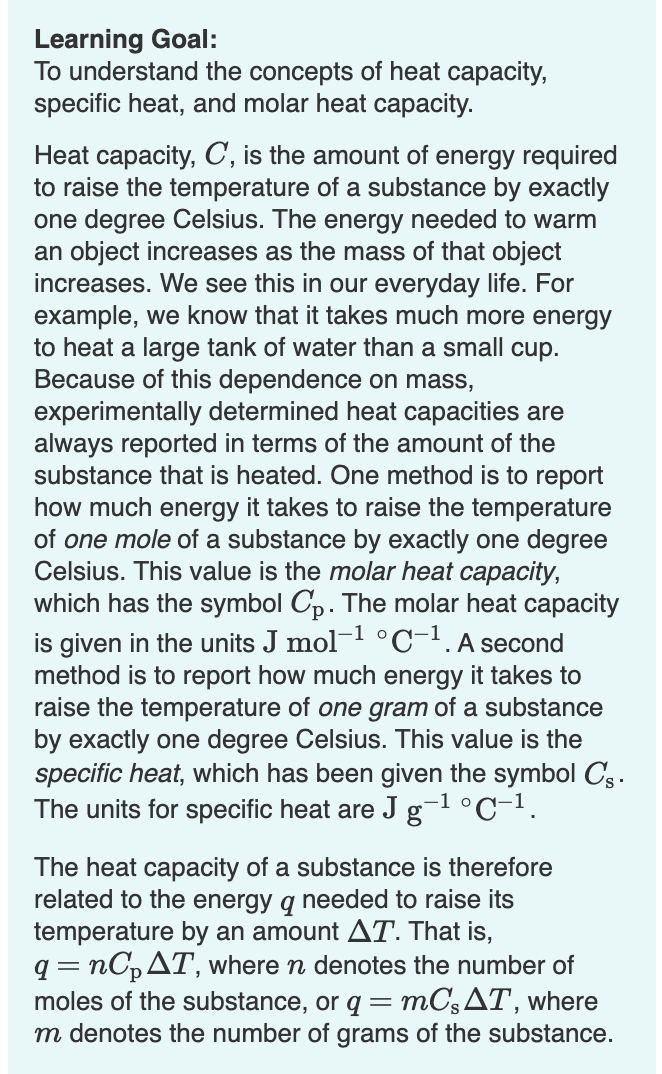
Transcribed Image Text:Learning Goal:
To understand the concepts of heat capacity,
specific heat, and molar heat capacity.
Heat capacity, C, is the amount of energy required
to raise the temperature of a substance by exactly
one degree Celsius. The energy needed to warm
an object increases as the mass of that object
increases. We see this in our everyday life. For
example, we know that it takes much more energy
to heat a large tank of water than a small cup.
Because of this dependence on mass,
experimentally determined heat capacities are
always reported in terms of the amount of the
substance that is heated. One method is to report
how much energy it takes to raise the temperature
of one mole of a substance by exactly one degree
Celsius. This value is the molar heat capacity,
which has the symbol Cp. The molar heat capacity
is given in the units J mol- °C-1.A second
method is to report how much energy it takes to
raise the temperature of one gram of a substance
by exactly one degree Celsius. This value is the
specific heat, which has been given the symbol Cs.
The units for specific heat are J g-l°C-1.
The heat capacity of a substance is therefore
related to the energy q needed to raise its
temperature by an amount AT. That is,
nC,AT, where n denotes the number of
MC3AT, where
moles of the substance, or q =
m denotes the number of grams of the substance.
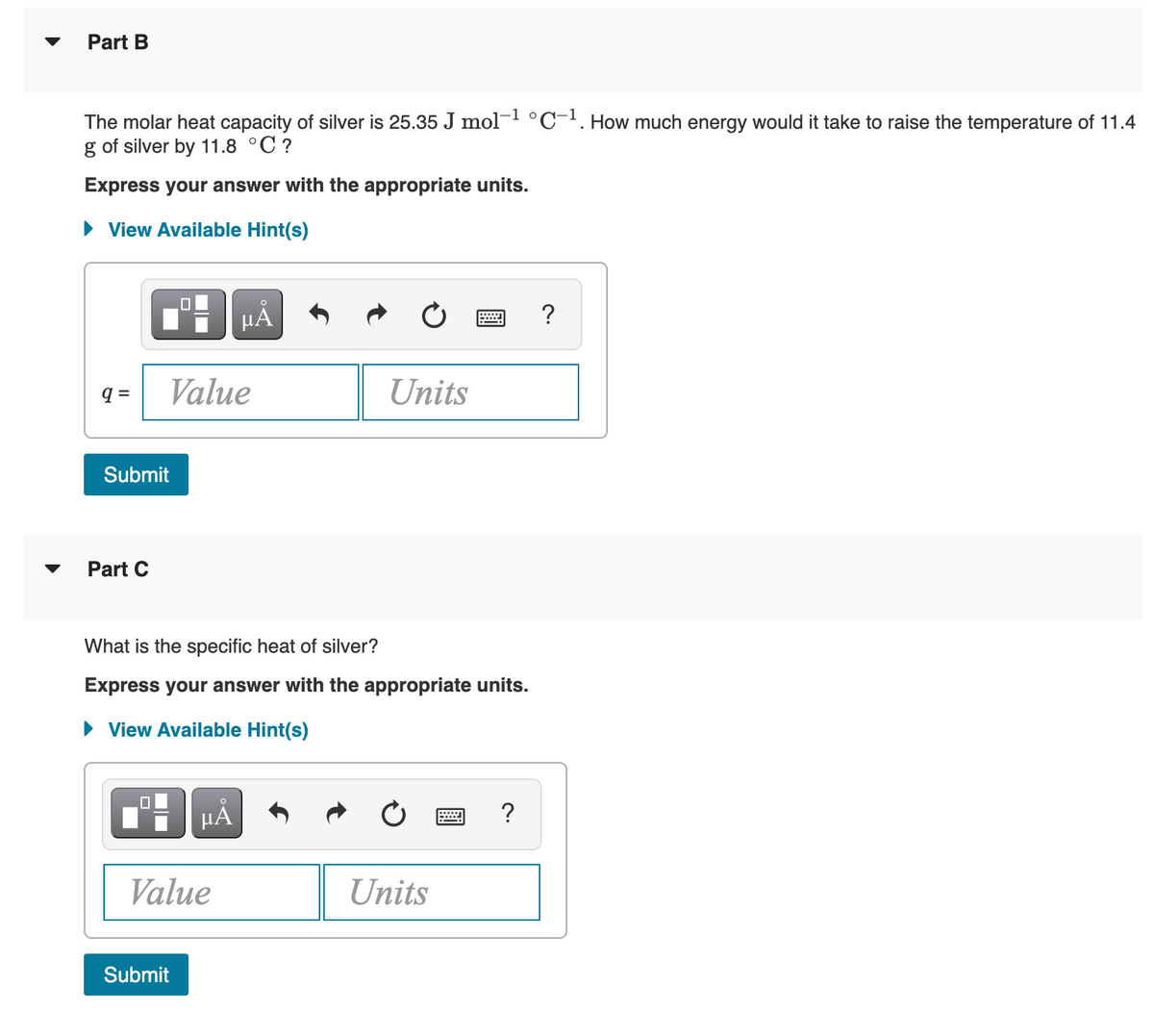
Transcribed Image Text:Part B
The molar heat capacity of silver is 25.35 J mol- °C-!. How much energy would it take to raise the temperature of 11.4
g of silver by 11.8 °C ?
Express your answer with the appropriate units.
• View Available Hint(s)
HẢ
?
q =
Value
Units
Submit
Part C
What is the specific heat of silver?
Express your answer with the appropriate units.
• View Available Hint(s)
HẢ
?
Value
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax