The most abur not fission. In a breeder reactor, however, a 238 U atom captures a neutron and emits two beta particles to make a fissionable isotope of plutonium, which can then be used as fuel in a nuclear reactor. Write the balanced nuclear equation. Express your answer as a nuclear equation. ΑΣΦΑΛΕΙ U- 238 Pl+2B 94 ? 238 92 A chemical reaction does not occur for this question That's not quite right. Please check your formatting No credit lost. Try again
The most abur not fission. In a breeder reactor, however, a 238 U atom captures a neutron and emits two beta particles to make a fissionable isotope of plutonium, which can then be used as fuel in a nuclear reactor. Write the balanced nuclear equation. Express your answer as a nuclear equation. ΑΣΦΑΛΕΙ U- 238 Pl+2B 94 ? 238 92 A chemical reaction does not occur for this question That's not quite right. Please check your formatting No credit lost. Try again
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 76QRT
Related questions
Question
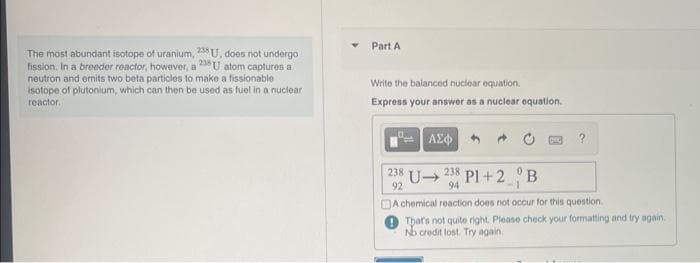
Transcribed Image Text:The most abundant isotope of uranium, 238 U, does not undergo
fission. In a breeder reactor, however, a 238 U atom captures a
neutron and emits two beta particles to make a fissionable
isotope of plutonium, which can then be used as fuel in a nuclear
reactor.
Part A
Write the balanced nuclear equation.
Express your answer as a nuclear equation.
ΑΣΦ
238
92
DOC
?
U➜
238 Pl+2 B
94
-1
A chemical reaction does not occur for this question.
That's not quite right. Please check your formatting and try again.
No credit lost. Try again.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning