The oxalate anion, C,042- has a C-C bond and two outer oxygen atoms bonded to each carbon atom. Determine the Lewis structure of this anion, including all resonance structures. What is the geometry about the carbon atoms, and how many atoms contribute p orbitals to the delocalized n system? Determine the Lewis structure of this anion, including all resonance structures. O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: 2- 2- O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: CHC CHC O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving two resonance structures:
The oxalate anion, C,042- has a C-C bond and two outer oxygen atoms bonded to each carbon atom. Determine the Lewis structure of this anion, including all resonance structures. What is the geometry about the carbon atoms, and how many atoms contribute p orbitals to the delocalized n system? Determine the Lewis structure of this anion, including all resonance structures. O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: 2- 2- O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures: CHC CHC O There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving two resonance structures:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 53IL: The sulfamate ion, H2NSO3, can be thought of as having been formed from the amide ion, NH2, and...
Related questions
Question
Please note there are 2 questions
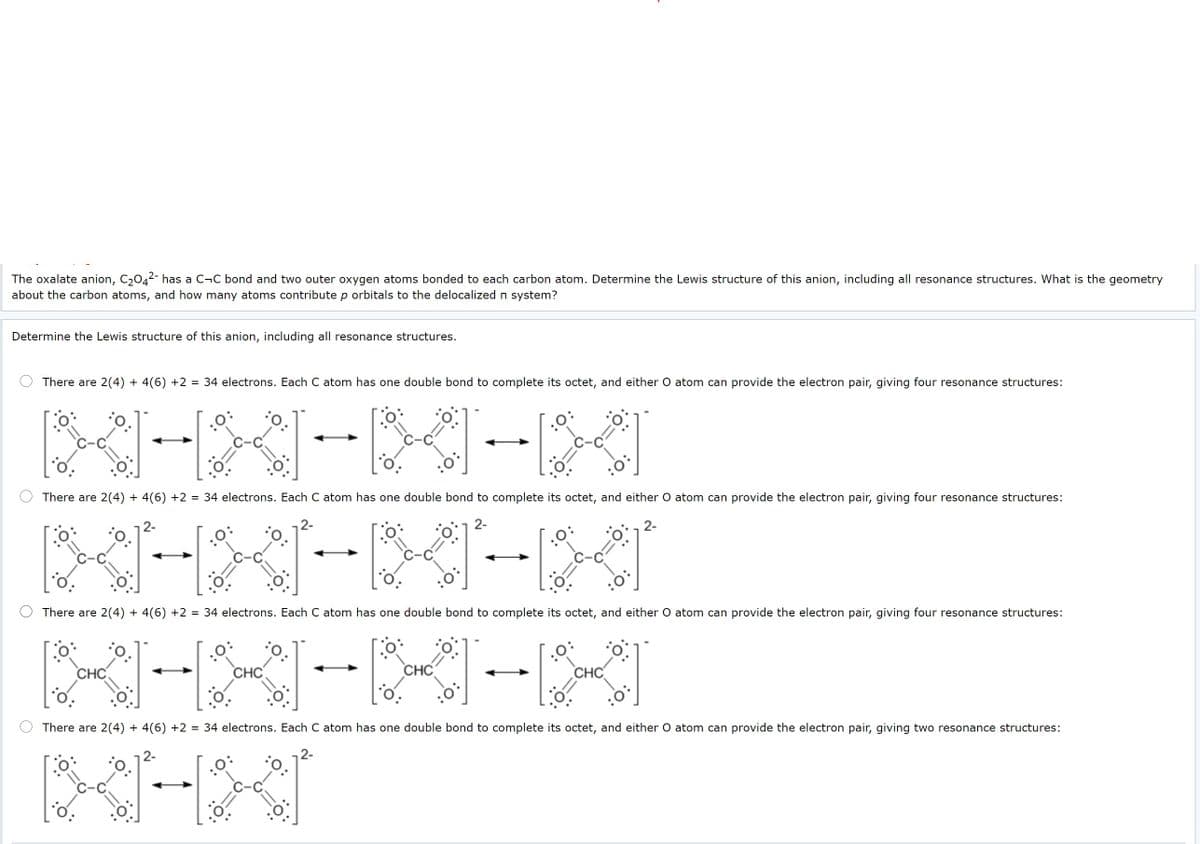
Transcribed Image Text:The oxalate anion, C,042- has a C¬C bond and two outer oxygen atoms bonded to each carbon atom. Determine the Lewis structure of this anion, including all resonance structures. What is the geometry
about the carbon atoms, and how many atoms contribute p orbitals to the delocalized n system?
Determine the Lewis structure of this anion, including all resonance structures.
There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures:
There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures:
2-
2-
2-
2-
There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving four resonance structures:
CHC
CHC
CHC
CHC
There are 2(4) + 4(6) +2 = 34 electrons. Each C atom has one double bond to complete its octet, and either O atom can provide the electron pair, giving two resonance structures:
2-
2-

Transcribed Image Text:What is the geometry about the carbon atoms, and how many atoms contribute p orbitals to the delocalized n system?
The geometry around each carbon atom is linear. All six atoms contribute p orbitals to the n system, which extends over the entire structure.
The geometry around each carbon atom is trigonal planar. All five atoms contribute p orbitals to the n system, which extends over the entire structure.
The geometry around each carbon atom is tetrahedral. All four atoms contribute p orbitals to the n system, which extends over the entire structure.
The geometry around each carbon atom is trigonal planar. All six atoms contribute p orbitals to the n system, which extends over the entire structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning