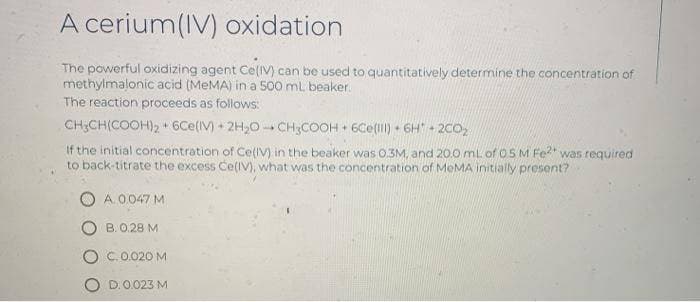
The powerful oxidizing agent Ce(V) can be used to quantitatively determine the concentration of methylmalonic acid (MEMA) in a 500 mL beaker. The reaction proceeds as follows: CH;CH(COOH)2 + 6Ce(IV)+ 2H,0 CH;COOH + 6Ce() • 6H + 2CO, If the initial concentration of CelIV) in the beaker was 03M, and 20.0 mL of 05 M Fe was required to back-titrate the excess Ce(IV), what was the concentration of MeMA initially present? O A.0047 M O B.0.28 M O C.0.020 M D.0.023 M
The powerful oxidizing agent Ce(V) can be used to quantitatively determine the concentration of methylmalonic acid (MEMA) in a 500 mL beaker. The reaction proceeds as follows: CH;CH(COOH)2 + 6Ce(IV)+ 2H,0 CH;COOH + 6Ce() • 6H + 2CO, If the initial concentration of CelIV) in the beaker was 03M, and 20.0 mL of 05 M Fe was required to back-titrate the excess Ce(IV), what was the concentration of MeMA initially present? O A.0047 M O B.0.28 M O C.0.020 M D.0.023 M
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter13: Electrochemistry
Section: Chapter Questions
Problem 13.101PAE
Related questions
Question
Transcribed Image Text:A cerium(IV) oxidation
The powerful oxidizing agent Ce(IV) can be used to quantitatively determine the concentration of
methylmalonic acid (MEMA) in a 500 mL beaker.
The reaction proceeds as follows:
CH;CH(COOH), 6Ce(IV) + 2H,0 - CH;COOH + 6Ce(l) 6H 2CO,
14
If the initial concentration of CelIV) in the beaker was 0.3M, and 20.0 mL of OSM Fe?* was required
to back-titrate the excess Ce(IV), what was the concentration of MeMA initially present?
A.0 047 M
B. 0.28 M
C.0.020 M
O D.0.023M

Transcribed Image Text:Reduction Potentials of Metal lon Complexes
Metal ions in a high oxidation state are often found complexed to anions in solution as opposed to
being "naked" aquo ions.
For example, complexation of Ce with the perchlorate anion CIO, shifts the standard reduction
potential of Ce*|Ce3+ from 1.47 to 1.70 V in acidic solution.
In marine environments, trace levels of Fe are scavenged by microbially produced catecholates
such as enterobactin. The (Fe(catecholz complex is calculated to shift the Fe"IFE2" reduction
potential from 0.771 to as low as -2.55 V (see Rizvi et al, Monatsh Chem 2017).
What can be said about the interactigns between perchlorate, catecholate and their respective
metal lons?
O A Perchlorate stabilizes Ce and catecholate stabilizes Fe?
O B. Perchlorate stabilizes Ce and catecholate stabilizes Fe
O C. Perchlorate stabilizes Ce* and catecholate stabilizes Fe
D. Perchlorate stabilizes Ce* and catecholate stabilizes Fe?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning