The product of the two provided equations (with Z = 1) is the ground state wave function for hydrogen. Consider that the radius of a proton is R0 = 10-15 m. For the ground state wave function for hydrogen, find the probabilty of finding the electron inside the proton (essentially within a sphere of the proton's radius). (Hint: make the integral for this problem easier by noting that R0 << a0
The product of the two provided equations (with Z = 1) is the ground state wave function for hydrogen. Consider that the radius of a proton is R0 = 10-15 m. For the ground state wave function for hydrogen, find the probabilty of finding the electron inside the proton (essentially within a sphere of the proton's radius). (Hint: make the integral for this problem easier by noting that R0 << a0
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 15CQ: Is it possible for to be smaller than unity? `
Related questions
Question
The product of the two provided equations (with Z = 1) is the ground state wave function for hydrogen.
Consider that the radius of a proton is R0 = 10-15 m. For the ground state wave function for hydrogen, find the probabilty of finding the electron inside the proton (essentially within a sphere of the proton's radius).
(Hint: make the integral for this problem easier by noting that R0 << a0.
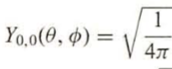
Transcribed Image Text:Yo,o(0,0) =
= √
1
4π
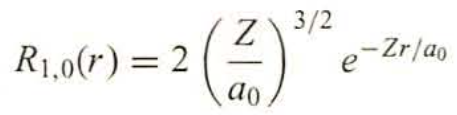
Transcribed Image Text:R₁,0(r) = 2
Z 3/2
ao
e-Zr/ao
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University