The pV diagram in (Figure 1) shows the cycle followed by the gas in an ideal-gas refrigerator whose coefficient of performance is 1.5. The work done by the system during two adiabatic processes is shown. Figure P 4 Adiabats 1 of 1 > W₁ = -119 J W, = 79 J V ▼ ✓ Correct Part F What is Win for one cycle of this heat engine? Express your answer with the appropriate units. ►View Available Hint(s) Win = 119 μÅ Submit Previous Answers Request Answer Part G Complete previous part(s) J X Incorrect; Try Again; One attempt remaining Part H Complete previous part(s) Provide Feedback ?
The pV diagram in (Figure 1) shows the cycle followed by the gas in an ideal-gas refrigerator whose coefficient of performance is 1.5. The work done by the system during two adiabatic processes is shown. Figure P 4 Adiabats 1 of 1 > W₁ = -119 J W, = 79 J V ▼ ✓ Correct Part F What is Win for one cycle of this heat engine? Express your answer with the appropriate units. ►View Available Hint(s) Win = 119 μÅ Submit Previous Answers Request Answer Part G Complete previous part(s) J X Incorrect; Try Again; One attempt remaining Part H Complete previous part(s) Provide Feedback ?
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 79AP: Consider the process shown below. During steps AB and BC, 3600 J and 2400 J of heat, respectively,...
Related questions
Question
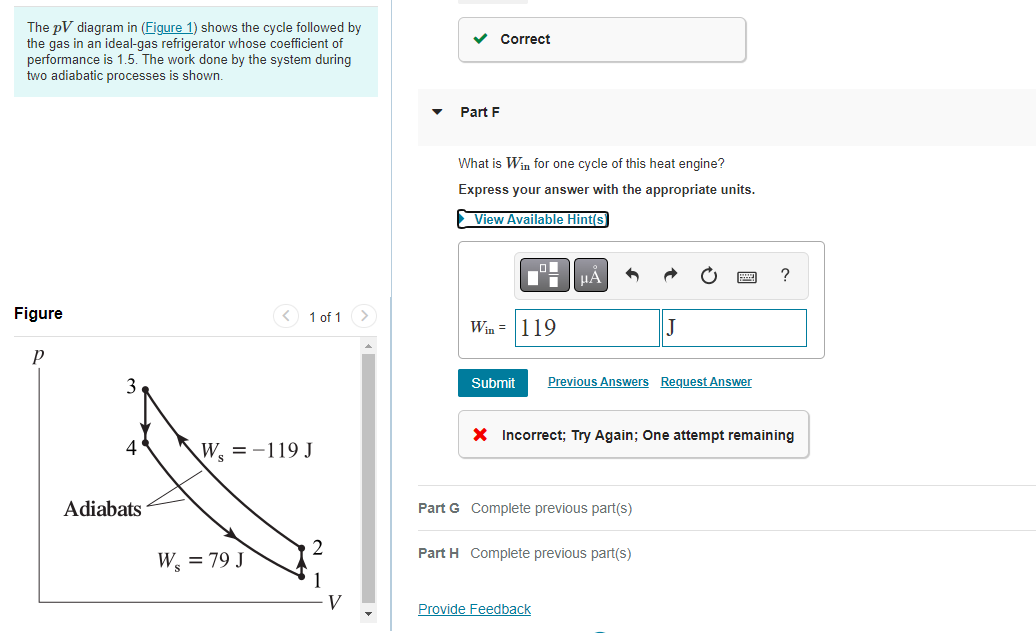
Transcribed Image Text:The pV diagram in (Figure 1) shows the cycle followed by
the gas in an ideal-gas refrigerator whose coefficient of
performance is 1.5. The work done by the system during
two adiabatic processes is shown.
Figure
P
4
Adiabats
1 of 1 >
W₁ = -119 J
W, = 79 J
V
▼
✓ Correct
Part F
What is Win for one cycle of this heat engine?
Express your answer with the appropriate units.
View Available Hint(s)
Win = 119
μÅ
Submit Previous Answers Request Answer
Part G Complete previous part(s)
J
X Incorrect; Try Again; One attempt remaining
Part H Complete previous part(s)
Provide Feedback
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College