The radius and the length of a cylindrical metal are 5.00 cm and 20.00 cm at 25°C, respectively. After it absorbed 9000 J of heat, the temperature raises from 25°C to 85°C and its length expands to 20.02 cm. Then, the cylinder was immersed to the water covered with insulator. The water initial temperature was 25°C and with mass 500 g. (Specific heat capacity of water is 4200 Jkg-1°C-1)(Heat loss through the container and cover is negligible.) Determine (a) the volume of the cylinder at 85°c. (b) the heat capacity of the metal. (c) the equilibrium temperature of water after the cylindrical metal was immersed to the water.
The radius and the length of a cylindrical metal are 5.00 cm and 20.00 cm at 25°C, respectively. After it absorbed 9000 J of heat, the temperature raises from 25°C to 85°C and its length expands to 20.02 cm. Then, the cylinder was immersed to the water covered with insulator. The water initial temperature was 25°C and with mass 500 g. (Specific heat capacity of water is 4200 Jkg-1°C-1)(Heat loss through the container and cover is negligible.) Determine (a) the volume of the cylinder at 85°c. (b) the heat capacity of the metal. (c) the equilibrium temperature of water after the cylindrical metal was immersed to the water.
Mathematics For Machine Technology
8th Edition
ISBN:9781337798310
Author:Peterson, John.
Publisher:Peterson, John.
Chapter65: Achievement Review—section Six
Section: Chapter Questions
Problem 55AR: Solve these prism and cylinder exercises. Where necessary, round the answers to 2 decimal places...
Related questions
Question
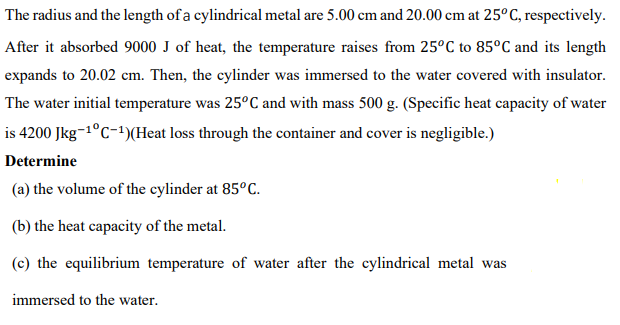
Transcribed Image Text:The radius and the length of a cylindrical metal are 5.00 cm and 20.00 cm at 25°C, respectively.
After it absorbed 9000 J of heat, the temperature raises from 25°C to 85°C and its length
expands to 20.02 cm. Then, the cylinder was immersed to the water covered with insulator.
The water initial temperature was 25°C and with mass 500 g. (Specific heat capacity of water
is 4200 Jkg-1°C-1)(Heat loss through the container and cover is negligible.)
Determine
(a) the volume of the cylinder at 85°c.
(b) the heat capacity of the metal.
(c) the equilibrium temperature of water after the cylindrical metal was
immersed to the water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,