The reaction between copper and hydrochloric acid is best represented by which of the following? The following activity series may be helpful.
The reaction between copper and hydrochloric acid is best represented by which of the following? The following activity series may be helpful.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.79QE
Related questions
Question
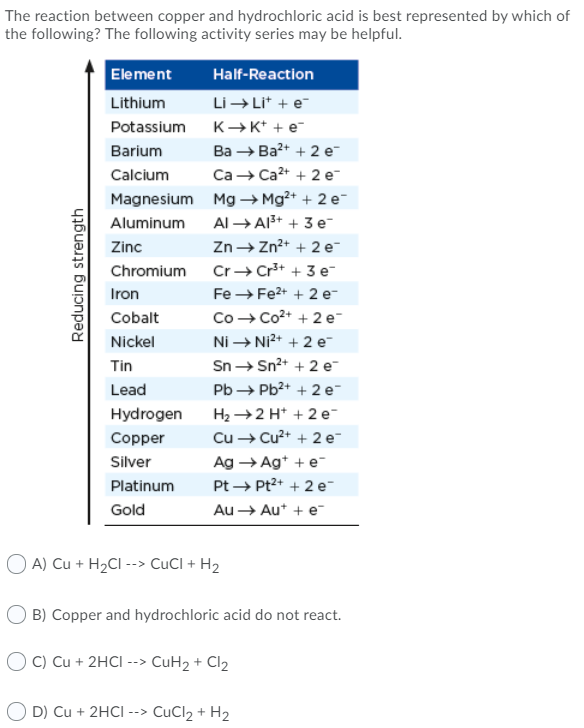
Transcribed Image Text:The reaction between copper and hydrochloric acid is best represented by which of
the following? The following activity series may be helpful.
Element
Half-Reaction
Lithium
Li → Li* + e
Potassium
K→K* + e-
Ва — Ва* + 2 е-
Са — Са2* + 2 ет
Barium
Calcium
Magnesium Mg → Mg²+ + 2 e-
Aluminum
Al → A3+ + 3 e-
Zinc
Zn→ Zn2+ + 2 e
Chromium
Cr+ Cr*+ + 3 e-
Iron
Fe → Fe2+ + 2 e-
Со — Со2+ + 2 ет
Ni → Ni2+ + 2 e-
Sn → Sn2+ + 2 e-
Cobalt
Nickel
Tin
Lead
Pb → Pb2+ + 2 e-
H2 →2 H* + 2 e
Cu → Cu2+ + 2 e
Hydrogen
Copper
Silver
Ag → Ag* + e
Platinum
Pt → Pt2+ + 2 e-
Gold
Au → Aut + e
A) Cu + H2CI --> CuCI + H2
O B) Copper and hydrochloric acid do not react.
O C) Cu + 2HCI
--> CuH2 + Cl2
D) Cu + 2HCI --> CuCl2 + H2
Reducing strength
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning