The reaction glyceraidehyde-3-phosphate NAD + P→ 1.3 diphosphoglycerale NADH + H has a standard reaction free energy (pH 7) of A.G 6.3 kJ mol ▼ Part A If the standard reduction potential of NAD +H+2e →NADH is 0.324 V, the reaction 1,3 dphosphoglycerate+ADP 3-phosphoglycero-ATP has a standard reaction free energy (pH 7) of A, G = -18.8 kJ/mol and the ATP hydrolysis reaction ATPH20 ADP- P₁ +H+ has a standard reaction free energy (pH 73 af-31.0 kamot, calculane the standard reduction poiantial for the reaction 3 phosphegyre+2+381 gyeraldehyde-3-phosphate + H₂0 Express your answer to three significant figures and include the appropriate units 2- PA .574 ↑ + Predoute 7
The reaction glyceraidehyde-3-phosphate NAD + P→ 1.3 diphosphoglycerale NADH + H has a standard reaction free energy (pH 7) of A.G 6.3 kJ mol ▼ Part A If the standard reduction potential of NAD +H+2e →NADH is 0.324 V, the reaction 1,3 dphosphoglycerate+ADP 3-phosphoglycero-ATP has a standard reaction free energy (pH 7) of A, G = -18.8 kJ/mol and the ATP hydrolysis reaction ATPH20 ADP- P₁ +H+ has a standard reaction free energy (pH 73 af-31.0 kamot, calculane the standard reduction poiantial for the reaction 3 phosphegyre+2+381 gyeraldehyde-3-phosphate + H₂0 Express your answer to three significant figures and include the appropriate units 2- PA .574 ↑ + Predoute 7
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.14QAP
Related questions
Question
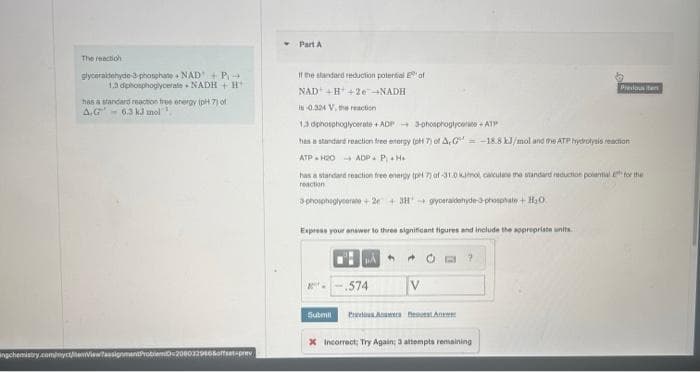
Transcribed Image Text:The reaction
glyceraldehyde-3-phosphate
NAD + P→
1.3 diphosphoglycerate NADH+H
has a standard reaction free energy (pH 7) of
A.G 6.3 kJ mol
ngchemistry.com/peycenView/assic
Soffset-prev
Part A
If the standard reduction potential of
NAD +H+2e →NADH
is 0.324 V, the reaction
1,3 dphosphoglycerate+ADP
3-phosphoglyce+ATP
has a standard reaction free energy (pH 7) of A,G= -18.8 kJ/mol and the ATP hydrolysis reaction
ATP H20 ADP PH
has a standard reaction free energy (pH 7) af-31.0 Kmo, cacutane the standard reduction polantial for the
reaction
phosphaglyser+2+31+ gyoeraldehyde-3-phosphate + H₂O
Express your answer to three significant figures and include the appropriate units.
Submi
PA
.574
Previous Ar
V
* Incorrect; Try Again; 3 attempts remaining
Predous ten
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 17 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
