The substance ammonia has the following properties: normal melting point: 195.4 K normal boiling point: 239.8 K 5.9×10-2 atm, 195.3 K triple point: critical point: 111.5 atm, 405.5 K A sample of ammonia at a pressure of 1.00 atm and a temperature of 209.6 K is cooled at constant pressure to a temperature of 197.5 K. Which of the following are true? Choose all that apply O No phase change will occur. O The final state of the substance is a liquid. O The liquid initially present will vaporize. O The final state of the substance is a solid. O The sample is initially a liquid.
The substance ammonia has the following properties: normal melting point: 195.4 K normal boiling point: 239.8 K 5.9×10-2 atm, 195.3 K triple point: critical point: 111.5 atm, 405.5 K A sample of ammonia at a pressure of 1.00 atm and a temperature of 209.6 K is cooled at constant pressure to a temperature of 197.5 K. Which of the following are true? Choose all that apply O No phase change will occur. O The final state of the substance is a liquid. O The liquid initially present will vaporize. O The final state of the substance is a solid. O The sample is initially a liquid.
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 108E: Consider the following data for xenon: Triple point: 121C, 280 torr Normal melting point: 112C...
Related questions
Question
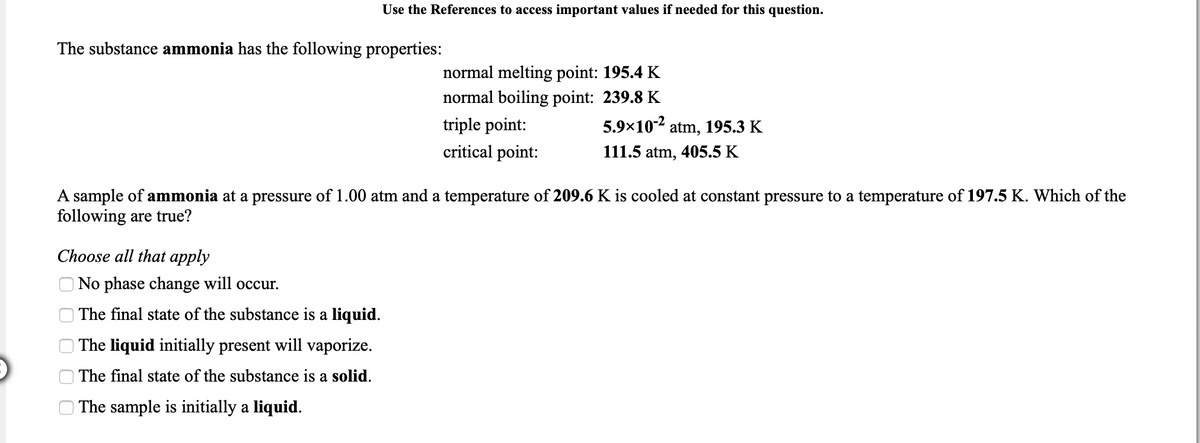
Transcribed Image Text:Use the References to access important values if needed for this question.
The substance ammonia has the following properties:
normal melting point: 195.4 K
normal boiling point: 239.8 K
triple point:
5.9×10-2
atm, 195.3 K
critical point:
111.5 atm, 405.5 K
A sample of ammonia at a pressure of 1.00 atm and a temperature of 209.6 K is cooled at constant pressure to a temperature of 197.5 K. Which of the
following are true?
Choose all that apply
No phase change will occur.
The final state of the substance is a liquid.
| The liquid initially present will vaporize.
The final state of the substance is a solid.
The sample is initially a liquid.
O O O

Transcribed Image Text:Use the References to access important values if needed for this question.
The substance fluorine has the following properties:
normal melting point: 53.5 K
normal boiling point: 85.0 K
triple point:
1.6×10-4 atm, 53.4 K
critical point:
55 atm, 144.1 K
At temperatures above 144.1 K and pressures above 55 atm, F2 is v
solid
liquid
F2 does not exist as a liquid at pressures below
atm.
gas
supercritical fluid
F2 is a
O at 11.4 atm and 42.5 K.
F2 is a
O at 1.00 atm and 73.9 K.
F2 is a
at 1.60×10-4 atm and 95.9 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning