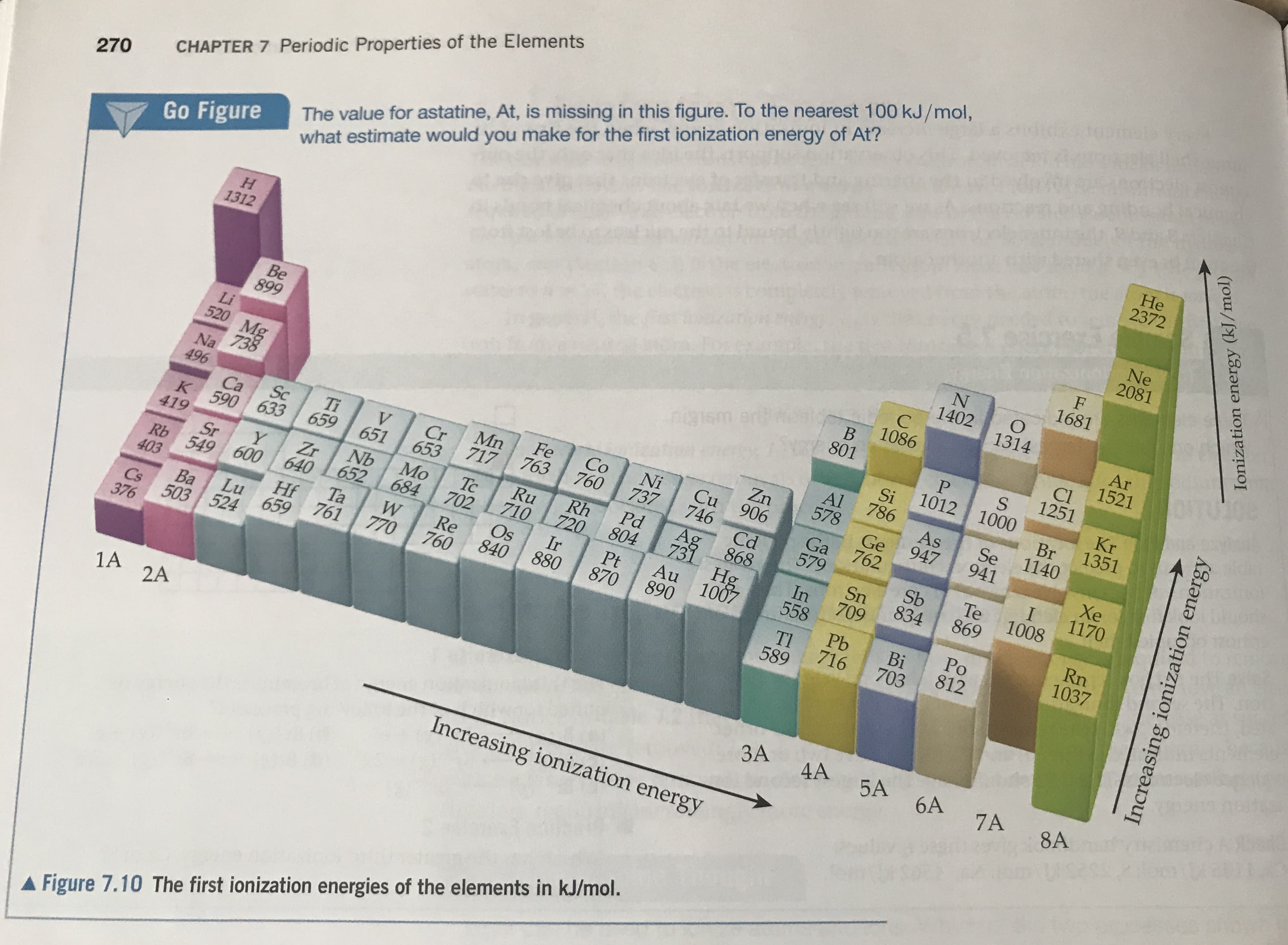
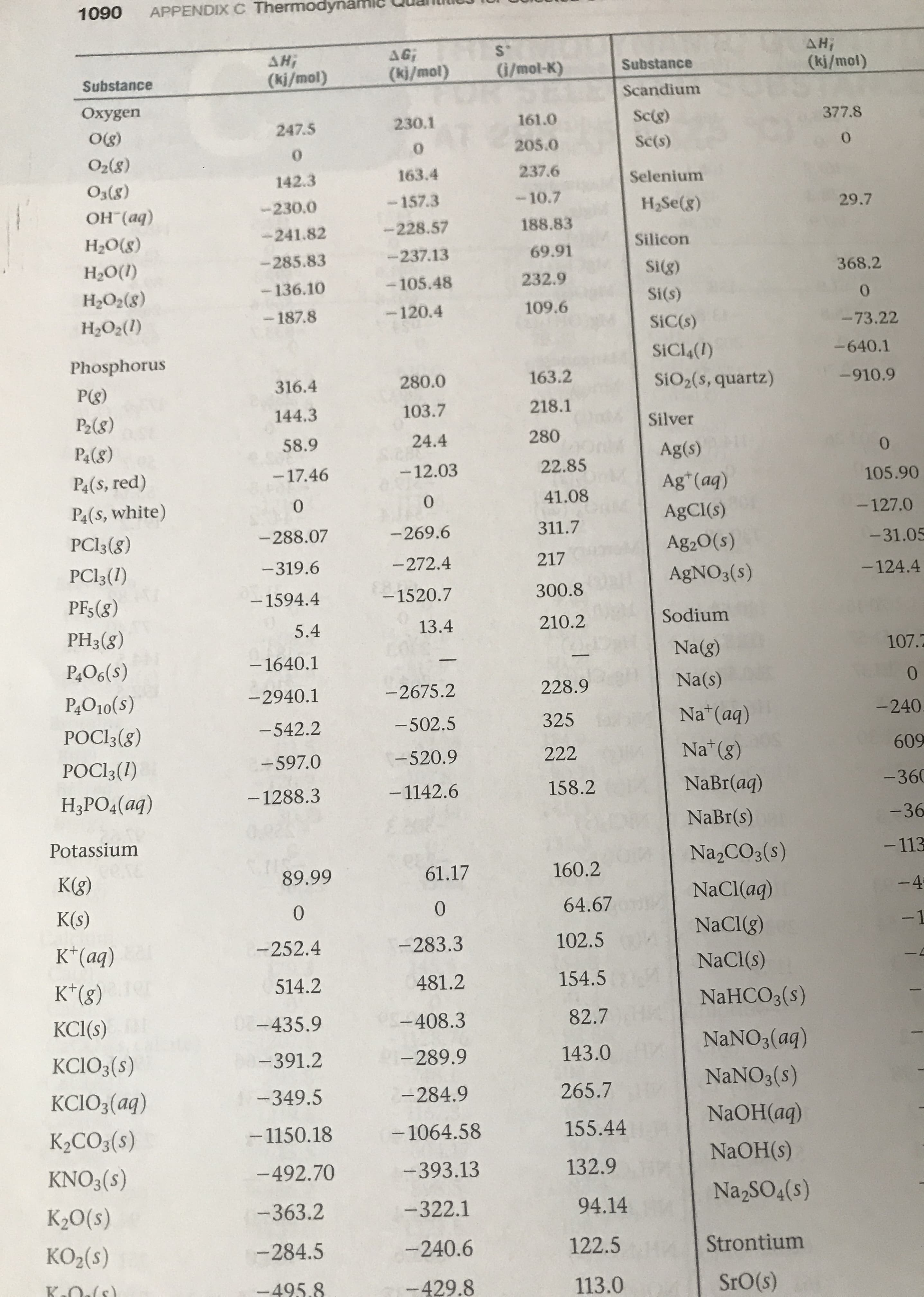
The value for astatine, At, is missing in this figure. To the nearest 100 kJ/mol, what estimate would you make for the first ionization energy of At? CHAPTER 7 Periodic Properties of the Elements He 2372 270 Go Figure Ne 2081 H 1312 F 1681 N 1402 O 1314 C Ar 1521 Be 899 1086 B ue wstthu 801 Cl 1251 Li 520 Mg 738 S 1012 1000 Kr 1351 Si 786 Br 1140 Al 578 Na As 947 Zn Cu 906 746 Se 941 496 Fe 763 Co 760 Ni 737 Mn 717 Ge 762 Ti 659 Sc V Cr Ca K 590 633 Xe 1170 Ga 579 651 653 Cd I 1008 Pd 804 Ag Rh 720 419 Ru 710 Ir Te 869 Sb 834 868 731 Hg 1007 Mo 684 Tc 702 Nb Zr 652 Y Sn 709 Sr Rb 549 600 640 Ta 761 In 558 Rn 1037 Au 890 Pt 870 Os 403 Re 760 Hf 659 880 Po 812 840 Pb 716 770 Lu Ba 503 524 Bi 703 Tl 589 Cs 376 1A 2A 3A 4A 5A 6A 7A 8A Increasing ionization energy AFigure 7.10 The first ionization energies of the elements in kJ/mol. Increasing ionization energy Ionization energy (kJ/mol) APPENDIX C Thermodynaml 1090 AH S. AG AH Substance (ki/mol) G/mot-K) (ki/mol) (ki/mol) Substance Scandium Oxygen Sc(g) 377.8 161.0 230.1 247.5 Og) Sc(s) 205.0 0 0 O2(8) 237.6 163.4 142.3 Selenium (8)EO OH (aq) -10.7 157.3 230.0 H2SE(8) 29.7 188.83 -228.57 -241.82 H2O(8) Silicon 69.91 -237.13 -285.83 Н.О() Si(g) 368.2 232.9 -105.48 136.10 НаО,(8) Si(s) 0 109.6 -120.4 -187.8 HаО:(1) SiC(s) - 73.22 SiCl4(I) -640.1 Phosphorus 163.2 280.0 SiO2(s, quartz) 316.4 -910.9 P(g) 218.1 103.7 144.3 P2(8) Silver 280 24.4 58.9 P&(&) 0 Ag(s) 22.85 -12.03 -17.46 P4(s, red) 105.90 Ag (aq) 41.08 0 0 P4(s, white) -127.0 AgCl(s) 311.7 -269.6 -288.07 PCI3(g) - 31.05 Ag2O(s) 217 - 272.4 - 319.6 PCI3(1) - 124.4 AGNO3(s) 300.8 - 1520.7 -1594.4 PFs(8) Sodium 210.2 13.4 5.4 PH3(8) 0% Na(g) 107.2 -1640.1 P&O6(s) 0 Na(s) 228.9 -2675.2 - 2940.1 P4O10(s) Na*(aq) - 240 325 -502.5 - 542.2 POCI3(g) Na (8) 609 222 -520.9 -597.0 POCI3(I) NaBr(aq) -360 158.2 -1142.6 -1288.3 Н,РО4(ад) -36 NaBr(s) Potassium -113 Na2CO3(s) cere K(g) 160.2 61.17 89.99 -4 NaCl(aq) 64.67 0 K(s) 0 -1 NaClg) 102.5 -283.3 -252.4 K*(aq) NaCl(s) 154.5 K*(8) 514.2 481.2 NAHCO3(s) 07-435.9 82.7 KCl(s) - 408.3 NANO3(aq) KCIO3(s) 143.0 -289.9 -391.2 NANO3(s) KCIO3(aq) -349.5 265.7 -284.9 NAOH(aq) - 1150.18 155.44 -1064.58 K2CO3(s) NAOH(s) 132.9 -393.13 KNO3(s) -492.70 Na2SO4(s) К0() -363.2 -322.1 94.14 КO,(5) -240.6 -284.5 122.5 Strontium K Oals) SrO(s) -495.8 -429.8 113.0
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
The
O(g)+e-—>O-(g)
The lattice energy of K2O(s) is 2238kj/mol.Use these data along with data in Appendix C and figure 7.10 to calculate the “second electron affinity” of oxygen, corresponding to the reaction
O-(g)+e-—>O2-(g)
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images







