The vapor pressures of each component in a mixture of propanone (acetone, A) and trichloromethane (chloroform, C) were measured at 35°C with the following results: In the above, xC is molar fraction of compound C. Check whether the mixture conforms to Raoult’s law for the component in large excess and to Henry’s law for the minor component. Provide relevant graphs with calculations. Find the Henry’s law constant for each of the two substances. If the substantial difficulties are encountered in finding the Henry’s law constants, explain what these problems are and why they occur.
The vapor pressures of each component in a mixture of propanone (acetone, A) and trichloromethane (chloroform, C) were measured at 35°C with the following results: In the above, xC is molar fraction of compound C. Check whether the mixture conforms to Raoult’s law for the component in large excess and to Henry’s law for the minor component. Provide relevant graphs with calculations. Find the Henry’s law constant for each of the two substances. If the substantial difficulties are encountered in finding the Henry’s law constants, explain what these problems are and why they occur.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter19: The Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 87QRT: Assume that the radius of Earth is 6400 km, the crust is 50. km thick, the density of the crust is...
Related questions
Question
The vapor pressures of each component in a mixture of propanone (acetone, A) and trichloromethane (chloroform, C) were measured at 35°C with the following results:
In the above, xC is molar fraction of compound C.
Check whether the mixture conforms to Raoult’s law for the component in large excess and to Henry’s law for the minor component.
Provide relevant graphs with calculations.
Find the Henry’s law constant for each of the two substances.
If the substantial difficulties are encountered in finding the Henry’s law constants, explain what these problems are and why they occur.
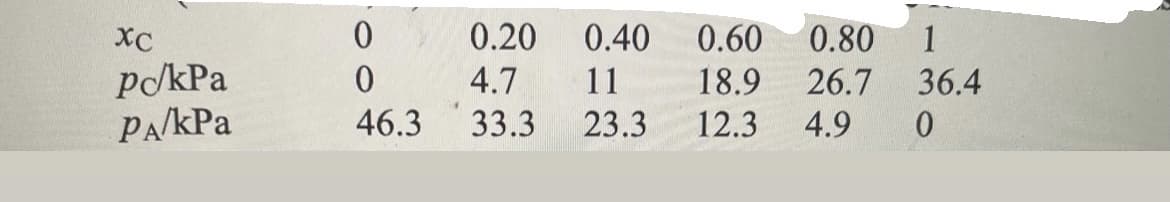
Transcribed Image Text:хс
Po/kPa
PA/KPa
0
0
46.3
0.20
0.40
0.60
0.80 1
4.7
11
18.9
26.7 36.4
33.3 23.3 12.3 4.9 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning