There are several chemical equilibria taking place in a solution of carbon dioxide in water: 1. CO₂(g) = CO₂(aq) 2. CO₂(aq) + H₂O(l) = H₂CO3(aq) 3. H₂CO3(aq) HCO3(aq) + H*(aq) 4. HCO3(aq) CO3² (aq) + H+ (aq) If OH- (from a strong base such as sodium hydroxide) is added to a solution of carbon dioxide, what happens to the OH and what effect will this have on the equilibria above? Select one: O O OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right. However, there is no H*(aq) involved in either the first or second reactions, so these equilibria will remain unaffected by the additon of OH(aq). OH-(aq) is not involved in any of the four equilibrium reactions that carbon dioxide participates in and so the OH(aq) will have no influence on the carbon dioxide equilibria Carbon dioxide dissolves and then reacts to produce the acidic carbonic acid molecule (H₂CO3). The H₂CO3 molecule is only present in acidic solutions and not in basic solutions. This means that added OH-(aq) which makes the solution basic, will stop the H2CO3 molecule from forming and so the first and second equilibrium reactions above will be pushed to the left and less carbon dioxide will dissolve in the solution. OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right which reduces the concentration of carbonic acid, which shifts the second reaction to the right, and then the first reaction shifts to the right so more CO₂(g) dissolves.
There are several chemical equilibria taking place in a solution of carbon dioxide in water: 1. CO₂(g) = CO₂(aq) 2. CO₂(aq) + H₂O(l) = H₂CO3(aq) 3. H₂CO3(aq) HCO3(aq) + H*(aq) 4. HCO3(aq) CO3² (aq) + H+ (aq) If OH- (from a strong base such as sodium hydroxide) is added to a solution of carbon dioxide, what happens to the OH and what effect will this have on the equilibria above? Select one: O O OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right. However, there is no H*(aq) involved in either the first or second reactions, so these equilibria will remain unaffected by the additon of OH(aq). OH-(aq) is not involved in any of the four equilibrium reactions that carbon dioxide participates in and so the OH(aq) will have no influence on the carbon dioxide equilibria Carbon dioxide dissolves and then reacts to produce the acidic carbonic acid molecule (H₂CO3). The H₂CO3 molecule is only present in acidic solutions and not in basic solutions. This means that added OH-(aq) which makes the solution basic, will stop the H2CO3 molecule from forming and so the first and second equilibrium reactions above will be pushed to the left and less carbon dioxide will dissolve in the solution. OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right which reduces the concentration of carbonic acid, which shifts the second reaction to the right, and then the first reaction shifts to the right so more CO₂(g) dissolves.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
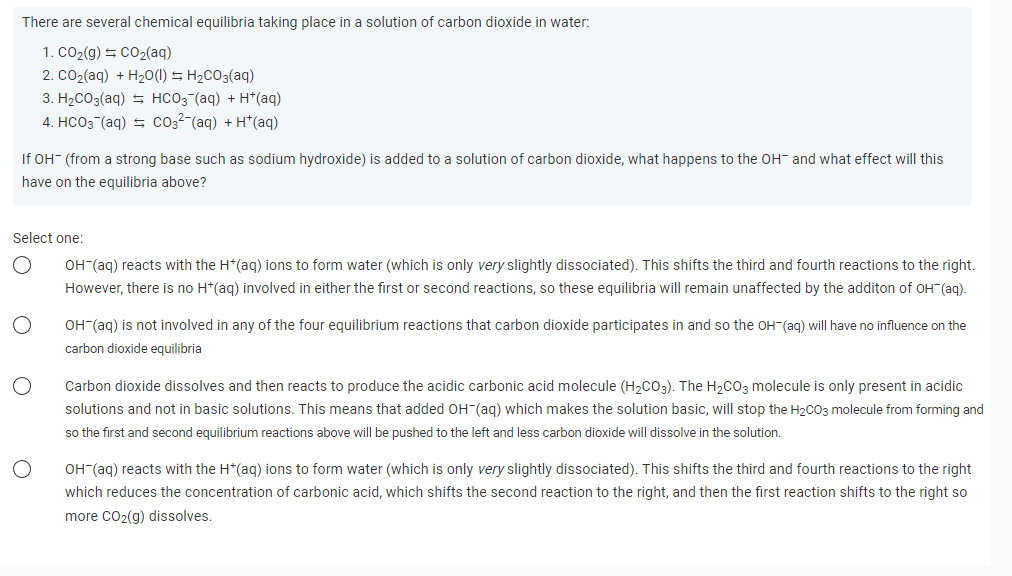
Transcribed Image Text:There are several chemical equilibria taking place in a solution of carbon dioxide in water:
1. CO₂(g) + CO₂(aq)
2. CO₂(aq) + H₂O(l) = H₂CO3(aq)
3. H₂CO3(aq) HCO3(aq) + H+ (aq)
4. HCO3(aq) = CO3²-(aq) + H*(aq)
If OH- (from a strong base such as sodium hydroxide) is added to a solution of carbon dioxide, what happens to the OH- and what effect will this
have on the equilibria above?
Select one:
OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right.
However, there is no H*(aq) involved in either the first or second reactions, so these equilibria will remain unaffected by the additon of OH¯(aq).
O
OH-(aq) is not involved in any of the four equilibrium reactions that carbon dioxide participates in and so the OH-(aq) will have no influence on the
carbon dioxide equilibria
Carbon dioxide dissolves and then reacts to produce the acidic carbonic acid molecule (H₂CO3). The H₂CO3 molecule is only present in acidic
solutions and not in basic solutions. This means that added OH(aq) which makes the solution basic, will stop the H₂CO3 molecule from forming and
so the first and second equilibrium reactions above will be pushed to the left and less carbon dioxide will dissolve in the solution.
OH-(aq) reacts with the H*(aq) ions to form water (which is only very slightly dissociated). This shifts the third and fourth reactions to the right
which reduces the concentration of carbonic acid, which shifts the second reaction to the right, and then the first reaction shifts to the right so
more CO₂(g) dissolves.

Transcribed Image Text:In part B of this experiment carbon dioxide gas was bubbled through a lime water (saturated calcium hydroxide) solution for several minutes.
At Step 8 the lime water solution was heated on a steam bath for about three minutes and a precipitate was observed to form.
From the options below, which give the equation(s) that accurate explain the observed results?
Select one:
O a. Ca²+ (aq) + CO3²-(aq) CaCO3(s)
O b. COz(aq) → CO2(g)
then
CO₂(aq) + Ca²+ (aq) + 2HO (aq) = CaCO3(s) + H₂O(aq)
O c. CO₂(aq) → CO₂ (s)
O d. CO₂(aq) → CO₂(g)
then
CaCO3(aq) + H₂O(aq) Ca(OH)2(s) + CO₂(aq)
Oe. CO₂(aq) = CO₂(g)
then
Ca²+ (aq) + 2HCO3(aq) = CaCO3(s) + CO₂(aq) + H₂O(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning