This is an experiment of a salt hydrate CuSo4 xH20 obtained during a practical. Use these values to calculate the moles crystal water and moles salt a hydrate present in the original sample.
This is an experiment of a salt hydrate CuSo4 xH20 obtained during a practical. Use these values to calculate the moles crystal water and moles salt a hydrate present in the original sample.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter2: Electrical Components And Circuits
Section: Chapter Questions
Problem 2.12QAP
Related questions
Question
This is an experiment of a salt hydrate CuSo4 xH20 obtained during a practical. Use these values to calculate the moles crystal water and moles salt a hydrate present in the original sample.
Transcribed Image Text:124
/ Chemie - Chemistry - 124
Practicals | Praktika
Practical Report 2| Praktiese Verslag 2
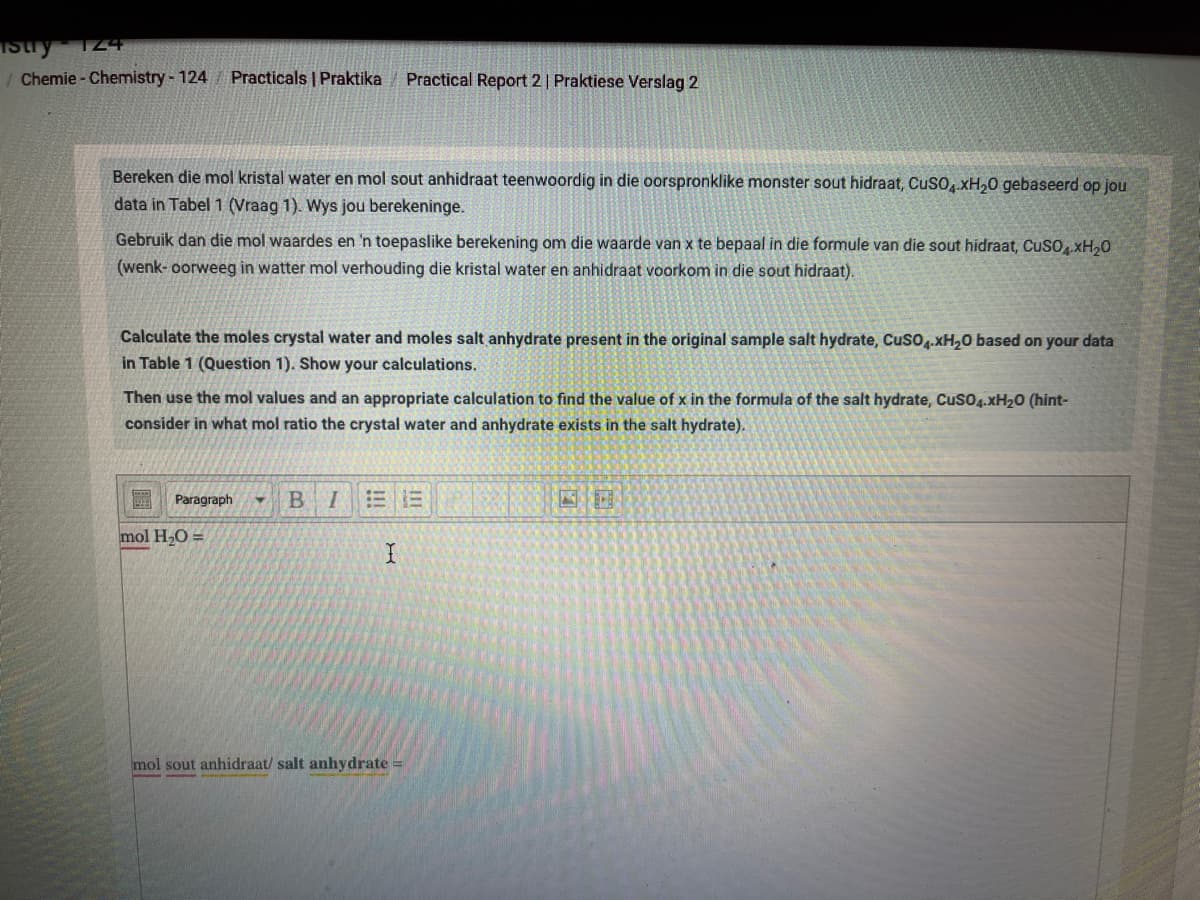
Bereken die mol kristal water en mol sout anhidraat teenwoordig in die oorspronklike monster sout hidraat, CusO, xH,0 gebaseerd op jou
data in Tabel 1 (Vraag 1). Wys jou berekeninge.
Gebruik dan die mol waardes en 'n toepaslike berekening om die waarde van x te bepaal in die formule van die sout hidraat, CuSOxH,0
(wenk- oorweeg in watter mol verhouding die kristal water en anhidraat voorkom in die sout hidraat).
Calculate the moles crystal water and moles salt anhydrate present in the original sample salt hydrate, Cuso.xH,0 based on your data
in Table 1 (Question 1). Show your calculations.
Then use the mol values and an appropriate calculation to find the value of x in the formula of the salt hydrate, Cuso4.XH20 (hint-
consider in what mol ratio the crystal water and anhydrate exists in the salt hydrate).
Paragraph
mol H,O =
mol sout anhidraat/ salt anhydrate =
Transcribed Image Text:TUDENTS - Go...
+ New Customers (...
E Policy for Registra.
Coachbit
t Coaching - Googl.
BI
S X| xE|三三
3 (12pt)
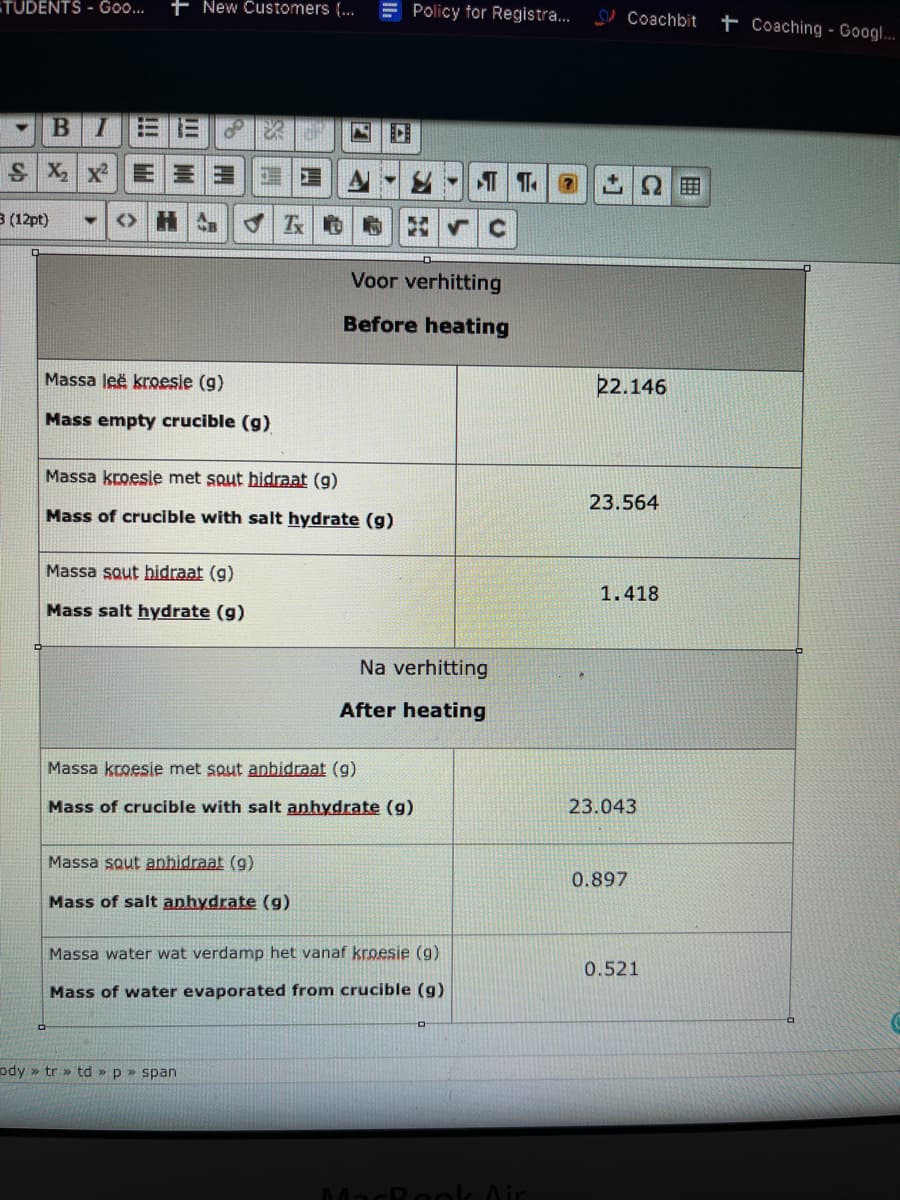
Voor verhitting
Before heating
Massa leë kroesie (g)
22.146
Mass empty crucible (g)
Massa kroesie met squt hidraat (g)
23.564
Mass of crucible with salt hydrate (g)
Massa sout hidraat (g)
1.418
Mass salt hydrate (g)
Na verhitting
After heating
Massa kroesie met sout anbidraat (g)
Mass of crucible with salt anhydrate (g)
23.043
Massa sout anbidraat (g)
0.897
Mass of salt anhydrate (g)
Massa water wat verdamp het vanaf kroesie (g)
0.521
Mass of water evaporated from crucible (g)
ody » tr » td » p » span
MacRook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning