This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel refining): Ni(CO)4 A chemical engineer has determined by measurements that there are 0.0939 moles of nickel in a sample of nickel tetracarbonyl. How many moles of oxygen are in the sample? Be sure your answer has the correct number of significant digits.
This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel refining): Ni(CO)4 A chemical engineer has determined by measurements that there are 0.0939 moles of nickel in a sample of nickel tetracarbonyl. How many moles of oxygen are in the sample? Be sure your answer has the correct number of significant digits.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter3: Equation, The Mole, And Chemical Formulas
Section: Chapter Questions
Problem 3.76QE
Related questions
Question
Please round to correct number of significant digits
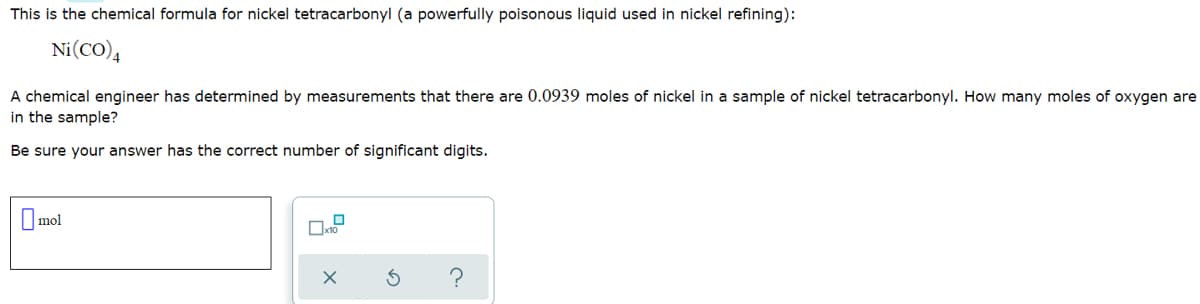
Transcribed Image Text:This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel refining):
Ni(CO),
A chemical engineer has determined by measurements that there are 0.0939 moles of nickel in a sample of nickel tetracarbonyl. How many moles of oxygen are
in the sample?
Be sure your answer has the correct number of significant digits.
O mol
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co