This problem will require you to upload images of your work for credit. Label this "Problem C." I have a bowl of warm water (2 kg at 40 C) and I plop 0.5 kg of dry ice into it to produce that cool Halloween fog! The dry ice immediantly sucks heat out of the water and sublimates into a gas. Once all the CO, has left. will some of the water be frozen? Justify, in your work, the answer you choose. (Note: The specific heat of liquid water is 4182 and the latent heat of sublimalon of dry ice is 199.000 kg kg 'C O Yes, my math shows that the final temperature of the water wants to go below 0 degrees. Therefore, some will freeze, O No, my math shows the final temperature of the water will be greater than 0 degrees,
This problem will require you to upload images of your work for credit. Label this "Problem C." I have a bowl of warm water (2 kg at 40 C) and I plop 0.5 kg of dry ice into it to produce that cool Halloween fog! The dry ice immediantly sucks heat out of the water and sublimates into a gas. Once all the CO, has left. will some of the water be frozen? Justify, in your work, the answer you choose. (Note: The specific heat of liquid water is 4182 and the latent heat of sublimalon of dry ice is 199.000 kg kg 'C O Yes, my math shows that the final temperature of the water wants to go below 0 degrees. Therefore, some will freeze, O No, my math shows the final temperature of the water will be greater than 0 degrees,
Related questions
Question
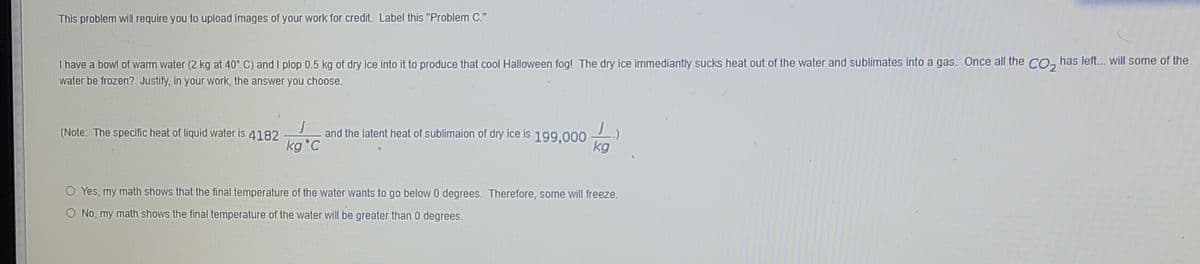
Transcribed Image Text:This problem will require you to upload images of your work for credit. Label this "Problem C."
I have a bowl of warm water (2 kg at 40° C) and I plop 0.5 kg of dry ice into it to produce that cool Halloween fog! The dry ice immediantly sucks heat out of the water and sublimates into a gas. Once all the CO, has left.. will some of the
water be frozen? Justify, in your work, the answer you choose.
and the latent heat of sublimaion of dry ice is 199.000
kg
(Note: The specific heat of liquid water is 4182
kg °C
Yes, my math shows that the final temperature of the water wants to go below 0 degrees. Therefore, some will freeze.
O No, my math shows the final temperature of the water will be greater than 0 degrees.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
