This questions will take you through an estimate of the number of gas molecules in a single breath and then nvestigate what the chances are that in any breath you take, at least one atom in any given breath you cake was also in the dying gasp of Cleopatra. We first estimate the number of atoms in a breath of air by considering that a breath of air has a volume of approximately 500 cubic centimeters. A cubic meter of air contains 2.5 x 1025 molecules. How many molecules, then are in one breath of air? (Use scientific notation.) Now, we want to estimate the number of breaths of air in the atmosphere of Earth. To do this, we first want to consider the volume of the Earth's atmosphere. Let's approximate this as a box with a side of length approximately h = 10 kilometers and a base equal to the surface area of the Earth (A = 5 x 10° square kilometers) so that the volume V = Ah. While this may seem like it's a strange "box", this is actually a very good approximation of the total volume of the atmosphere. What is this total volume in
This questions will take you through an estimate of the number of gas molecules in a single breath and then nvestigate what the chances are that in any breath you take, at least one atom in any given breath you cake was also in the dying gasp of Cleopatra. We first estimate the number of atoms in a breath of air by considering that a breath of air has a volume of approximately 500 cubic centimeters. A cubic meter of air contains 2.5 x 1025 molecules. How many molecules, then are in one breath of air? (Use scientific notation.) Now, we want to estimate the number of breaths of air in the atmosphere of Earth. To do this, we first want to consider the volume of the Earth's atmosphere. Let's approximate this as a box with a side of length approximately h = 10 kilometers and a base equal to the surface area of the Earth (A = 5 x 10° square kilometers) so that the volume V = Ah. While this may seem like it's a strange "box", this is actually a very good approximation of the total volume of the atmosphere. What is this total volume in
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:Elaine N. Marieb, Katja N. Hoehn
Chapter1: The Human Body: An Orientation
Section: Chapter Questions
Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,...
Related questions
Question
Transcribed Image Text:Question 7
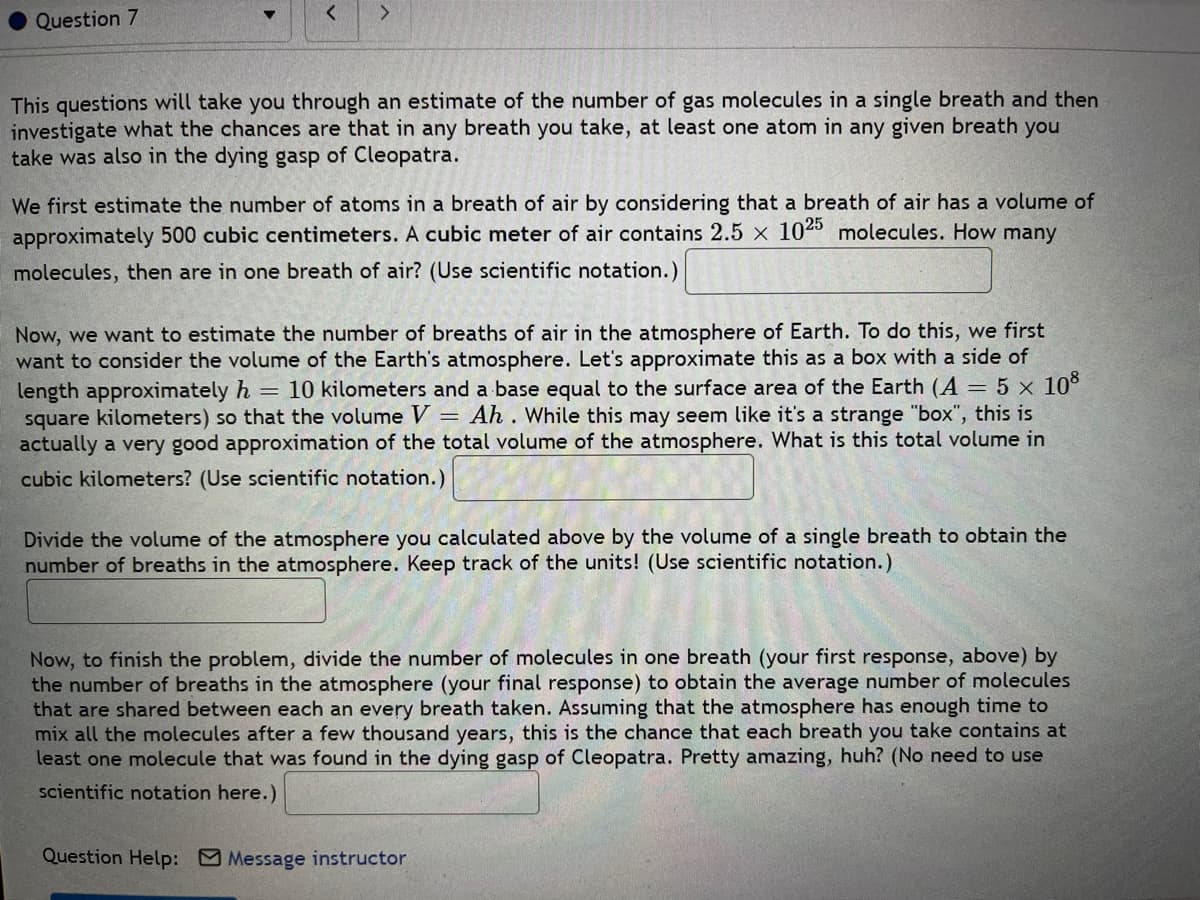
This questions will take you through an estimate of the number of gas molecules in a single breath and then
investigate what the chances are that in any breath you take, at least one atom in any given breath you
take was also in the dying gasp of Cleopatra.
We first estimate the number of atoms in a breath of air by considering that a breath of air has a volume of
approximately 500 cubic centimeters. A cubic meter of air contains 2.5 x 1025 molecules. How many
molecules, then are in one breath of air? (Use scientific notation.)
Now, we want to estimate the number of breaths of air in the atmosphere of Earth. To do this, we first
want to consider the volume of the Earth's atmosphere. Let's approximate this as a box with a side of
length approximately h = 10 kilometers and a base equal to the surface area of the Earth (A = 5 x 10°
square kilometers) so that the volume = Ah . While this may seem like it's a strange "box", this is
actually a very good approximation of the total volume of the atmosphere. What is this total volume in
cubic kilometers? (Use scientific notation.)
Divide the volume of the atmosphere you calculated above by the volume of a single breath to obtain the
number of breaths in the atmosphere. Keep track of the units! (Use scientific notation.)
Now, to finish the problem, divide the number of molecules in one breath (your first response, above) by
the number of breaths in the atmosphere (your final response) to obtain the average number of molecules
that are shared between each an every breath taken. Assuming that the atmosphere has enough time to
mix all the molecules after a few thousand years, this is the chance that each breath you take contains at
least one molecule that was found in the dying gasp of Cleopatra. Pretty amazing, huh? (No need to use
scientific notation here.)
Question Help: Message instructor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:
9780815344322
Author:
Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:
W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:
9781260159363
Author:
Martin, Terry R., Prentice-craver, Cynthia
Publisher:
McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:
9781260231700
Author:
Sylvia S. Mader, Michael Windelspecht
Publisher:
McGraw Hill Education