Ti(s)+2 Cl,(g)→TİCI 4(1) a certain temperature, a chemist finds that a 6.3 L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium has the lowing composition: compound amount dh Ti 1.04 g Cl2 2.34 g TICI4 4.49 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. %3D |Accessibility Tems of Use Privacy Chesk,
Ti(s)+2 Cl,(g)→TİCI 4(1) a certain temperature, a chemist finds that a 6.3 L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium has the lowing composition: compound amount dh Ti 1.04 g Cl2 2.34 g TICI4 4.49 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. %3D |Accessibility Tems of Use Privacy Chesk,
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.37PAE: Again the experiment in Exercise 12.33 was redesigned. This time, 0.15 mol each of N, and O2 was...
Related questions
Question
Transcribed Image Text:ab report A
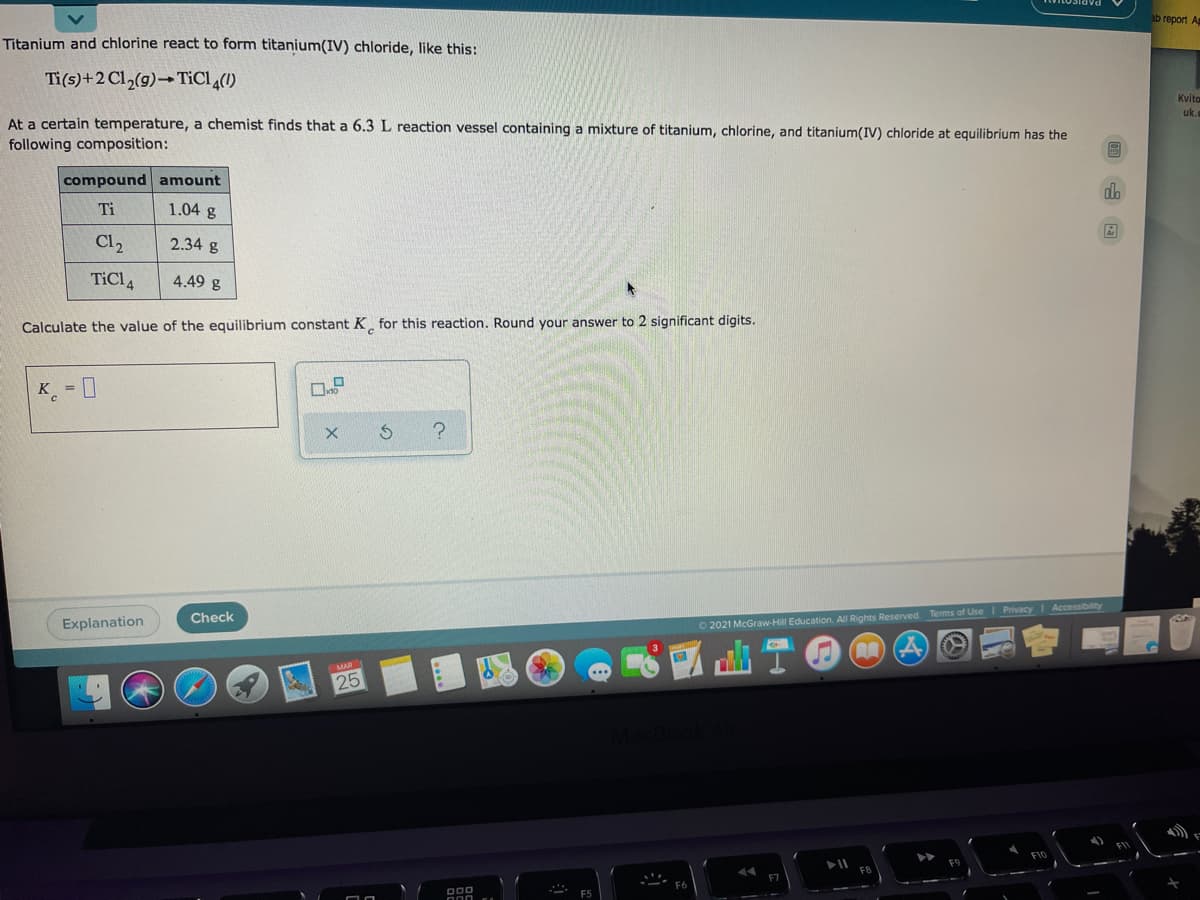
Titanium and chlorine react to form titanium(IV) chloride, like this:
Ti(s)+2 Cl,(g)→ TICI(1)
Kvita
uk.
At a certain temperature, a chemist finds that a 6.3 L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium has the
following composition:
compound amount
Ti
1.04 g
Cl2
2.34 g
TiCl 4
4.49 g
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
K_ = 0
%3D
Check
Explanation
2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy I Accessibility
25
D00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
