to bring water=M2XC2X(1-T,)Q Describe what would happef to the water in Question 2 if you continued to transfer heat energy at a constant rate even after the ice had melted. Sketch the temperature history on the axes below. Indicate on your graph the time when the water begins to boil. 100 Time (min) Temperature (°C)
to bring water=M2XC2X(1-T,)Q Describe what would happef to the water in Question 2 if you continued to transfer heat energy at a constant rate even after the ice had melted. Sketch the temperature history on the axes below. Indicate on your graph the time when the water begins to boil. 100 Time (min) Temperature (°C)
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter14: Heat And Heat Transfer Methods
Section: Chapter Questions
Problem 41PE: One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a...
Related questions
Question
100%
Question 6 is based on the question 2 so in case you need it
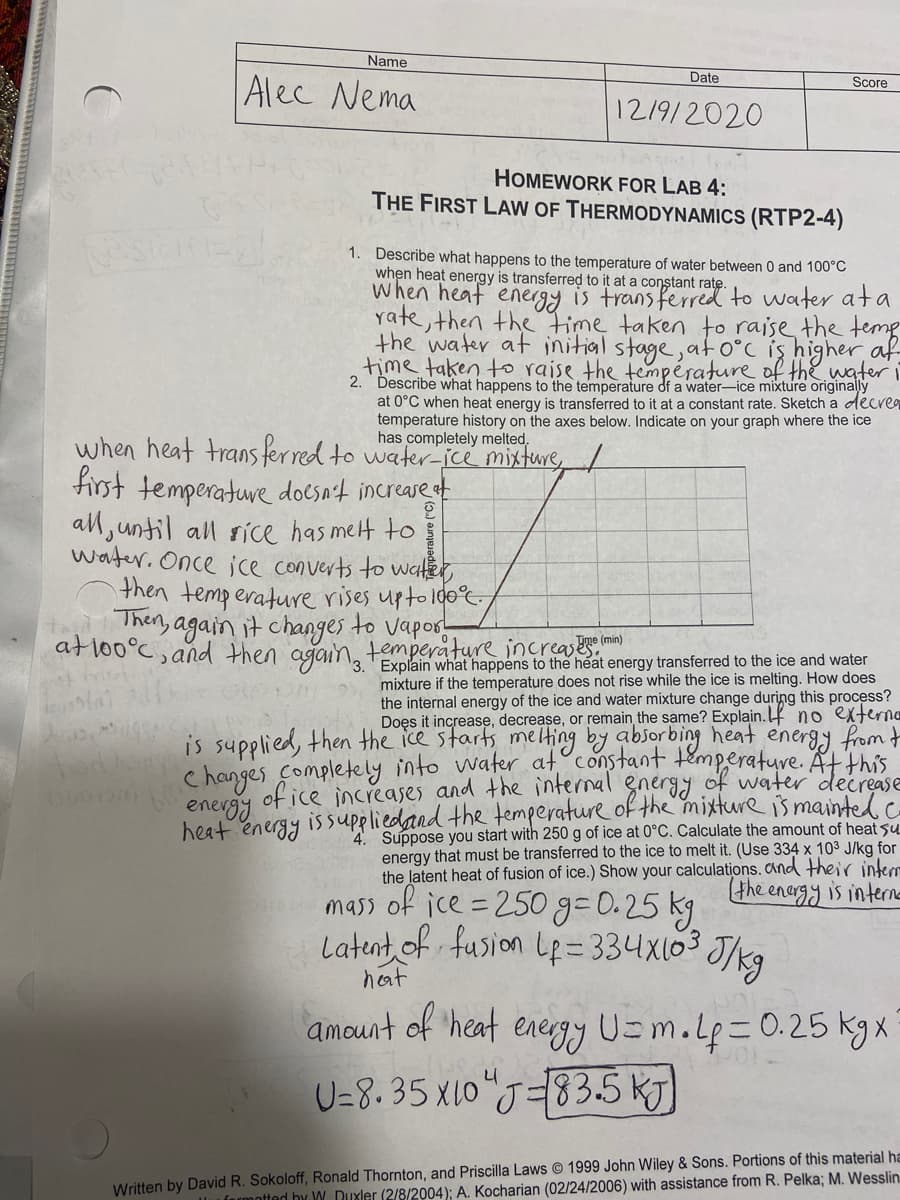
Transcribed Image Text:Name
Date
Alec Nema
Score
12/9/2020
HOMEWORK FOR LAB 4:
THE FIRST LAW OF THERMODYNAMICS (RTP2-4)
1. Describe what happens to the temperature of water between 0 and 100°C
when heat energy is transferred to it at a constant rate.
When heaf energy is trans ferred to water ata
rate, then the time taken to raise the temp
the water at initial stage,ato°c iş higher af
time taken to raise the température of thewateri
2. Describe what happens to the temperature df a water-ice mixture originally
at 0°C when heat energy is transferred to it at a constant rate. Sketch a decre9
temperature history on the axes below. Indicate on your graph where the ice
has completely melted,
when heat trans ferred to water-jce mixture
first temperature docsn't increase t
all, until all sice has melt to
water. Once jce converts to water,
then temperature rises upto 100°
Then, again it changes to vapos-
atlo0°C, and then aganng tolain what happens to the heat energy transferred to the ice and water
Tme (min)
temperature increases
mixture if the temperature does not rise while the ice is melting. How does
the internal energy of the ice and water mixture change during this process?
Doęs it increase, decrease, or remain the same? Explain. no externe
is supplied, then the ice starts melting by absorbing heat energy from t
hanges Completely into water at constant temperature. Atthis
of ice increases and the internal gneryy of water decrease
enevgy
heat energy issupeliedand the temperature of the mixture is mainted.c
4. Suppose you start with 250 g of ice at 0°C. Calculate the amount of heat su
energy that must be transferred to the ice to melt it. (Use 334 x 103 J/kg for
the latent heat of fusion of ice.) Show your calculations. and their interr
the enargy is intere
mass of ice =250 g=0.25 kg
Latent of fusion Le= 334x103 J/kg
heat
amount of 'heat
energy U=m.Lp=0.25 kgxX
U=8. 35 x10"J=83.5 KJ)
Written by David R. Sokoloff, Ronald Thornton, and Priscilla Laws 1999 John Wiley & Sons. Portions of this material ha
formotted hy W Duxler (2/8/2004); A. Kocharian (02/24/2006) with assistance from R. Pelka; M. Wesslin
![5. A mixture of 150 g of ice and 300 a of water is at 0°C. How many joules of
heat energy must be transferred to bring this mixture to a final temperature of
75°C? Assume that the heat energy transferred to the room is very small.
Show your calculations. [Use 334 x 103 J/kg (334 J/g) for the latent heat of
fusion of ice and 4190 J/kg°C (4.19 J/g°C) for the specific heat of water.]
Final temperature =75°C =T heat required to be sa
Inifial temperature of ice =T,
initial temperat ure of wat er Ti
Q1=S0100J+471
het required= mixL tm c (T+Fi)=Q2=91275,.
heat required to bring water=m2 XC2X(T-T,)=Q2 Q.
6. Describe what would happeR to the water in Question 2 if you continued to
transfer heat energy at a constant rate even after the ice had melted. Sketch
the temperature history on the axes below. Indicate on your graph the time
when the water begins to boil.
100
Time (min)
7. Explain what happens to the heat energy transferred to the water and steam
mixture if the temperature does not rise while the water is boiling. How does
the internal energy of the water and steam mixture change during this
process? Does it increase, decrease, or remain the same? Explain. NOw, wher
energy is supplied, then the ice starts meiting by ab
from the water. lce changes Completely intő water
At this condition, internal energy of ice increases
energy of water decreases.If the external energ
the Femperature is maintained constant, then the
is absorbed by both water and ice. Their internal
and then energy is the thermel potential eneryy
Suppose you start with 250 g of ice at 0°C. Calculate the amount of heat
Temperature (°C)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F95f0b7f6-52d2-4b69-91d7-019b1ba2e2f5%2F8cccaebb-f4b9-4959-9b58-ad82459b330a%2F2q93fxf_processed.jpeg&w=3840&q=75)
Transcribed Image Text:5. A mixture of 150 g of ice and 300 a of water is at 0°C. How many joules of
heat energy must be transferred to bring this mixture to a final temperature of
75°C? Assume that the heat energy transferred to the room is very small.
Show your calculations. [Use 334 x 103 J/kg (334 J/g) for the latent heat of
fusion of ice and 4190 J/kg°C (4.19 J/g°C) for the specific heat of water.]
Final temperature =75°C =T heat required to be sa
Inifial temperature of ice =T,
initial temperat ure of wat er Ti
Q1=S0100J+471
het required= mixL tm c (T+Fi)=Q2=91275,.
heat required to bring water=m2 XC2X(T-T,)=Q2 Q.
6. Describe what would happeR to the water in Question 2 if you continued to
transfer heat energy at a constant rate even after the ice had melted. Sketch
the temperature history on the axes below. Indicate on your graph the time
when the water begins to boil.
100
Time (min)
7. Explain what happens to the heat energy transferred to the water and steam
mixture if the temperature does not rise while the water is boiling. How does
the internal energy of the water and steam mixture change during this
process? Does it increase, decrease, or remain the same? Explain. NOw, wher
energy is supplied, then the ice starts meiting by ab
from the water. lce changes Completely intő water
At this condition, internal energy of ice increases
energy of water decreases.If the external energ
the Femperature is maintained constant, then the
is absorbed by both water and ice. Their internal
and then energy is the thermel potential eneryy
Suppose you start with 250 g of ice at 0°C. Calculate the amount of heat
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning